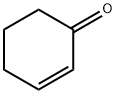
Applications of 2-Cyclohexen-1-one
2-Cyclohexen-1-one (2CHO) is a non-planar, sixmembered ring molecule for which conjugation between the C=O and C=C groups is expected to provide extra rigidity to the ring.Furthermore, the conjugation should make 2-Cyclohexen-1-one possible to treat the ring inversion process using a onedimensional potential energy function. 2-Cyclohexen-1-one is an organic compound which is a versatile intermediate used in the synthesis of a variety of chemical products such as pharmaceuticals and fragrances. 2-Cyclohexen-1-one is clear colorless liquid in pure state but a commercially available product is mostly yellowish liquid. 2-Cyclohexen-1-one is soluble in many solvents, such as alcohols, ethers, haloalkanes, esters, and also is miscible with polar aprotic solvents. 2-Cyclohexen-1-one reacts both ketones and alkenes. 2-Cyclohexen-1-one has an electron-poor carbon-carbon double bond as a typical representative of the α, β-unsaturated carbonyl compounds.
2-Cyclohexen-1-one appears as a colorless to pale yellow liquid with a pleasant odor. Less dense than water . Flash point 111°F. Vapors heavier than air. 2-Cyclohexen-1-one is used to make nylon, as a chemical reaction medium, and as a solvent.
Tricyclic keto diols have been synthesised by a new, three-step annulation procedure in which hydroxyenones, prepared by the coupling of 2-cyclohexen-1-one with aldehydes, are diastereoselectively epoxidised and the syn-epoxides cyclised with tin(IV) chloride[1].
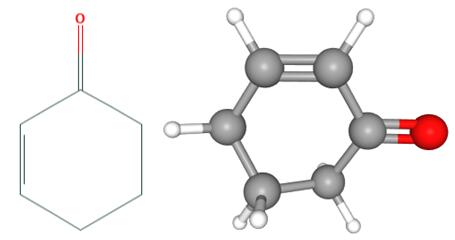
Fig 1. Chemical structure formula and three-dimensional structure of 2-Cyclohexen-1-one
Selective hydrogenation of 2-cyclohexen-1-one over Pt-MCM-41 proceeds at a very high rate and produces cyclohexanone with selectivity of 100% in a batch reactor[2].
The electrocatalytic hydrogenation (ECH) of 2-cyclohexen- 1 -one to cyclohexanone has been studied on various modified and generally expensive electrode materials[3].
2-Cyclohexen-1-one is a multifunctional electrophile for many addition reactions, including conjugate addition of an organic copper nucleophile, Michael addition to an enol silane, and phosphonium silylation.
2-cyclohexen-1-one is a widely used building block in organic synthesis chemistry, as 2-cyclohexen-1-one offers many different ways to extend molecular frameworks. Cyclohexenone is easily adapted to Michael addition with nucleophiles (such as enolates or silyl enol ethers) or, 2-cyclohexen-1-one could be employed by a Diels-Alder reaction with electron-rich dienes.
2-cyclohexen-1-one is a waste chemical stream constituent which may be subjected to ultimate disposal by controlled incineration[4].
References
[1] Marson C M , Benzies D W M , Hobson A D , et al. Convergent, stereocontrolled routes to hydroxylated tricyclic systems: a new annulation of 2-cyclohexen-1-one[J]. Journal of the Chemical Society, Chemical Communications, 1990, 21(21):1516-1518.
[2] Chatterjee M , Yokoyama T , Kawanami H , et al. An exceptionally rapid and selective hydrogenation of 2-cyclohexen-1-one in supercritical carbon dioxide[J]. Chemical Communications, 2009(6):701-703.
[3] J. Fournier, P. K. Wrona, A. Lasia. R. Lacasse, J. M.Lalancette, H. MCnard and L. Brossard. J. Ekwrroc~hen~.Sot. 139, 2372 (1992).
[4] Engineering Handbook for Hazardous Waste Incineration p.2.5 (1981) EPA 68-03-3025.
You may like
See also
Lastest Price from 2-Cyclohexen-1-one manufacturers
US $0.00-0.00/KG2025-04-21
- CAS:
- 930-68-7
- Min. Order:
- 25KG
- Purity:
- 99.8
- Supply Ability:
- 20ton
US $650.00/Kg2025-03-29
- CAS:
- 930-68-7
- Min. Order:
- 0.100Kg
- Purity:
- 98
- Supply Ability:
- 5000 Kg