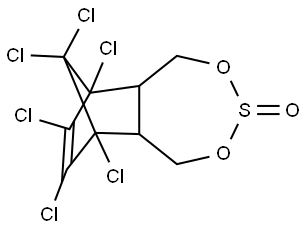
Endosulfan (70) [115-29-7], 6,7,8,9,10,10-
hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3,-
benzo-dioxathiepine 3-oxide (IUPAC) [The technical product
is a mixture of two isomers: α-endosulfan: 3α,5αβ,6α,
9α,9αβ (64–67%) (70a) and β-endosulfan 3α,5aα,6β,9β,
9aα, (29–32%) (70b)]; [959-98-8] (formerly [33213-66-
0]) (β-endosulfan);[33213-65-9] (formerly [891-86-1] and
[19670-15-6]) (β-endosulfan) is the adduct of hexachlorocyclopentadiene
and 1,4-dihydroxy-2-butene reacted further
with SOCl2 to produce 6,7,8,9,10,10-hexachloro-
1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzodioxa
thiepin-3-oxide. The technical product is a brownish solid,
mp 70–100 C, vapor pressure 1.3 mPa at 25 C, soluble in
petroleum solvents but having low solubility in water. It
consists of about four parts of α-isomer (mp 108 C, cis with
regard to the sulfite group) and one part of the β-isomer
(mp 206 C, trans with regard to the sulfite group). The
α-isomer, which is somewhat more insecticidal, is slowly
converted to the more stable β-isomer at high temperature,
and both isomers are oxidized slowly to endosulfan sulfate
[1031-07-8] (mp 181 C). In acid media, both isomers form
endosulfan diol [2157-19-9] (mp 203 C).