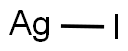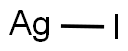Silver iodide (AgI) precipitates as a yellow, odor- and tasteless solid. Silver iodide acts as a very effective nucleus for the formation of ice crystals. Silver iodide has an important advantage over mercury as a subject for study of electrochemical properties of interfaces. It is also used as a solid lubricant for power contacts. It is also used as a local antiseptic.