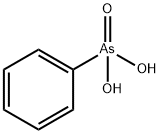
Arsonic acids (arsonic acid, phenylarsonic acid, p-hydroxyphenylarsonic acid,
arsanilic acid) react with the tetravalent metals of group IV of the periodic
system to give water-insoluble complexes of composition MA2 . The composition
of the precipitates is not exactly stoichiometric, and hence the precipitates are not
suitable for direct gravimetric analysis. Instead, the precipitates are transformed
by ignition to the corresponding metal oxides and weighed as such. The main
advantage of the application of these reagents is the possibility offered of the
selective determination of zirconium(IV), hafnium(IV) and titanium(IV) in the
presence of many other metals, such as zinc, manganese, nickel, cobalt, aluminium,
copper, calcium, magnesium and chromium. In practice they are utilized most
frequently for the determination of zirconium.
In strong mineral acidic media phenylarsonic acid forms water-insoluble precipitates
with zirconium and hafnium only. In highly concentrated acetic acid
solution the thorium phenylarsonate complex is also precipitated, which makes
possible the separation of thorium from rare earth metals.
The procedure gives satisfactory results in the presence of alkaline earth metals,
iron, aluminium, manganese, cobalt, nickel, copper, zinc and bismuth.