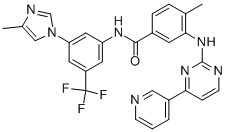
Nilotinib is the other second-generation Abl kinase inhibitor approved for treatment of patients with CML. Nilotinib has increased affinity for the Abl kinase compared with imatinib, binding with an improved topologic fit to the kinase site in its inactive form.Nilotinib is active in chronic and accelerated phase CML patients who have developed resistance to imatinib. As with dasatinib, nilotinib was compared with imatinib in a phase III randomized trial as initial therapy for chronic phase CML patients, with nilotinib achieving higher rates of complete cytogenetic and major molecular responses and with fewer cases of disease progression or clonal evolution in the nilotinib-treated cohorts. Survival outcomes were similar in all arms. Common adverse events with nilotinib included rash, gastrointestinal disturbances (nausea, vomiting, diarrhea), neutropenia, and thrombocytopenia. Pleural effusion and peripheral edema are less common than with dasatinib. In addition, QT prolongation and a risk of pancreatitis are serious side effects of nilotinib.