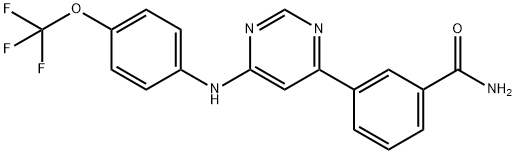
Bcr-Abl, a fusion protein with deregulated tyrosine kinase activity, is highly expressed in chronic myelogenous leukemia. GNF-2 is a Bcr-Abl inhibitor that binds to the myristoyl binding pocket, an allosteric site distant from the active site, stabilizing the inactive form of the kinase. Thus, it can selectively inhibit Bcr-Abl phosphorylation (IC50 = 267 nM) without affecting native c-Abl or a panel of 63 additional kinases. It demonstrates exclusive antiproliferative activity toward Bcr-Abl-transformed cells (IC50 = 138 nM), inducing apoptosis in these cells without cytotoxic consequences in nontransformed cells.