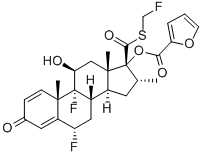
Fluticasone furoate is a new corticosteroid derivative launched as a
nasal spray for the treatment of seasonal and perennial allergic rhinitis in adults
and in children aged ≥2 years. It is structurally closely related to the previously
marketed intranasal corticosteroid fluticasone propionate (FP). Both of these
steroids contain the unusual S-fluoromethyl carbothioate group, which confers
high lipophilicity and hence enhanced binding and retention of the drug by the
nasal tissue. Additionally, the carbothioate group rapidly undergoes first-pass
metabolism by CYP3A4, thus minimizing systemic exposure of the parent drug.
Fluticasone furoate is a potent ligand for the glucocorticoid receptor (GR), with
a relative receptor affinity (RRA) of 2,989 with reference to dexamethasone RRA
of 100.
Following intranasal administration, most of the dose is eventually swallowed and undergoes incomplete absorption and extensive firstpass metabolism in the liver and gut, resulting in negligible systemic exposure. Pharmacokinetic studies using oral solution dosing and intravenous dosing show that at least 30% of fluticasone furoate is absorbed and then rapidly cleared from plasma. The most common adverse reactions (W1% incidence) included headache, epistatix, sinus and throat pain, nasal ulceration, back pain, pyrexia, and cough. Fluticasone furoate is chemically derived starting from a readily available corticosteroid, 6a,9a-difluoro-11b,17a-dihydroxy-16a-methyl-3-oxoandrosta-1,4-diene-17b-carboxylic acid, by first converting the carboxylic acid group to the corresponding carbothioic acid via activation with carbonyl diimidazole followed by reaction with hydrogen sulfide gas. Subsequently, selective acylation of the 17a-hydroxyl group with 2-furoyl chloride and alkylation of the 17b-carbothioic acid group with bromofluoromethane under basic conditions provides fluticasone furoate.