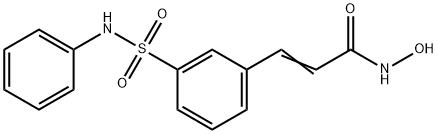
Belinostat is a drug which was developed by Spectrum Pharmaceuticals
and is currently marketed by Onxeo as Beleodaq®. The
drug, which received fast track designation by the United States
Food and Drug Administration (US FDA) and was approved for
the treatment of hematological malignancies and solid tumors
associated with peripheral T-cell lymphoma (PTCL) in 2014, is a
histone deacetylase (HDAC) inhibitor and is the third such treatment
to receive accelerated approval for PTCL, the others being
vorinostat (Zolinza®) and pralatrexate (Folotyn®). Although belinostat
was not yet approved in Europe as of August 2014, the
compound exhibits a safety profile considered to be acceptable
for HDAC inhibitors–less than 25% of patients reported adverse
effects and these most frequently were nausea, fatigue, pyrexia,
anemia, and emesis.