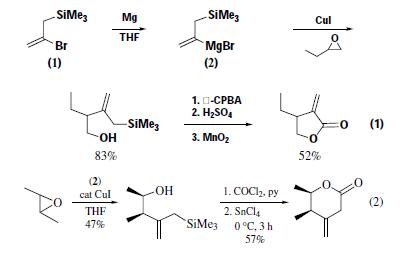
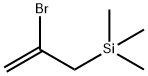
2-Bromo-3-trimethylsilyl-1-propene can be used as synthon for CH2=C?CH2TMS1–3 and CH2=CBrC?H2;2 for
synthesis of 1-trimethylsilylmethyl-substituted 1,3-butadienes.
The 1-trimethylsilylmethylvinyl
anion CH2=C(M)CH2TMS (2) (M = Li, Mg, Cu,
etc.), readily prepared from 2-bromo-3-trimethylsilyl-1-propene
(1) under typical conditions, allows the introduction of the
synthetically useful 1-trimethylsilylmethylvinyl group to a wide
variety of substrates. Ring opening of 1-butene oxide with the
Grignard reagent (2) (M = MgBr) in the presence of copper(I)
iodide gives only one regioisomer. Subsequent desilylative oxidation
of this allyl alcohol to α-methylene-γ-lactones provides
further utility of (1) as a 1-hydroxymethylvinyl anion equivalent,
i.e. CH2=?C?CH2OH (eq 1).Alternatively, the alcohol
from trans-2,3-epoxybutane provides a route to the unstable sixmembered
β,γ-unsaturated lactone (eq 2).The copper-catalyzed
1,4-addition to the typically unreactive mesityl oxide proceeds
smoothly. The versatility of the allylsilane moiety is again illustrated
in the ethylaluminum dichloride-induced cyclization of the
adduct to a tertiary cyclopentanol in high yield (eq 3).