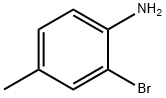
The N-(3, 5-Dichlorophenyl) naphthaldimine was obtained from the reaction of 2-hy- droxynaphthalene-l-carbaldehyde (0.01 mol) with a solution of 2-bromo-4-methylaniline (0.01 mol) in 40 ml of ethanol. Reaction of 2-bromo-4-methylaniline was quite clean, albeit 3,4-diaminotoluene was isolated just in 37% yield after flash chromatography on silica gel. The bromomethylquinoline starting material 16 was synthesized in two steps using an optimized process route by condensing 2-bromo-4-methylaniline.