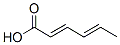
Sorbic acid
- русский язык имя
- английское имяSorbic acid
- CAS №22500-92-1
- CBNumberCB9954577
- ФормулаC6H8O2
- мольный вес0
- номер MDLMFCD00002703
- файл Mol22500-92-1.mol
| растворимость | Slightly soluble in water, freely soluble in ethanol (96 per cent). |
| Запах | characteristic |
| InChI | InChI=1S/C6H8O2/c1-2-3-4-5-6(7)8/h2-5H,1H3,(H,7,8)/b3-2+,5-4+ |
| ИнЧИКей | WSWCOQWTEOXDQX-MQQKCMAXSA-N |
| SMILES | C(O)(=O)/C=C/C=C/C |
| LogP | 1.268 (est) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P332+P313:При возникновении раздражения кожи: обратиться за медицинской помощью.
Sorbic acid химические свойства, назначение, производство
Химические свойства
Sorbic acid is a tasteless, white to yellow-white crystalline powder with a faint characteristic odor.Использование
Pharmaceutic aid (antimicrobial agent).Определение
ChEBI: A hexadienoic acid with double bonds at C-2 and C-4; it has four geometrical isomers, of which the trans,trans-form is naturally occurring.Методы производства
Naturally occurring sorbic acid may be extracted as the lactone (parasorbic acid) from the berries of the mountain ash Sorbus aucuparia L. (Fam. Rosaceae). Synthetically, sorbic acid may be prepared by the condensation of crotonaldehyde and ketene in the presence of boron trifluoride; by the condensation of crotonaldehyde and malonic acid in pyridine solution; or from 1,1,3,5- tetraalkoxyhexane. Fermentation of sorbaldehyde or sorbitol with bacteria in a culture medium has also been used.Фармацевтические приложения
Sorbic acid is an antimicrobial preservative with antibacterial and antifungal properties used in pharmaceuticals, foods, enteral preparations, and cosmetics. Generally, it is used at concentrations of 0.05–0.2% in oral and topical pharmaceutical formulations, especially those containing nonionic surfactants. Sorbic acid is also used with proteins, enzymes, gelatin, and vegetable gums. It has been shown to be an effective preservative for promethazine hydrochloride solutions in a concentration of 1 g/L.Sorbic acid has limited stability and activity against bacteria and is thus frequently used in combination with other antimicrobial preservatives or glycols, when synergistic effects appear to occur.
Безопасность
Sorbic acid is used as an antimicrobial preservative in oral and topical pharmaceutical formulations and is generally regarded as a nontoxic material. However, adverse reactions to sorbic acid and potassium sorbate, including irritant skin reactions and allergic hypersensitivity skin reactions (which are less frequent), have been reported.Other adverse reactions that have been reported include exfoliative dermatitis due to ointments that contain sorbic acid, and allergic conjunctivitis caused by contact lens solutions preserved with sorbic acid.
No adverse reactions have been described after systemic administration of sorbic acid, and it has been reported that it can be ingested safely by patients who are allergic to sorbic acid. However, perioral contact urticaria has been reported.
The WHO has set an estimated total acceptable daily intake for sorbic acid, calcium sorbate, potassium sorbate, and sodium sorbate, expressed as sorbic acid, at up to 25 mg/kg bodyweight. Animal toxicological studies have shown no mammalian carcinogenicity or teratogenicity for sorbic acid consumed at up to 10% of the diet.
LD50 (mouse, IP): 2.82 g/kg
LD50 (mouse, oral): 3.20 g/kg
LD50 (mouse, SC): 2.82 g/kg
LD50 (rat, oral): 7.36 g/kg
хранилище
Sorbic acid is sensitive to oxidation, particularly in the presence of light; oxidation occurs more readily in aqueous solution than in the solid form. Sorbic acid may be stabilized by phenolic antioxidants such as 0.02% propyl gallate.Sorbic acid is combustible when exposed to heat or flame. When heated to decomposition, it emits acrid smoke and irritating fumes. The bulk material should be stored in a well-closed container, protected from light, at a temperature not exceeding 40℃.
Несовместимости
Sorbic acid is incompatible with bases, oxidizing agents, and reducing agents. Some loss of antimicrobial activity occurs in the presence of nonionic surfactants and plastics. Oxidation is catalyzed by heavy-metal salts. Sorbic acid will also react with sulfur-containing amino acids, although this can be prevented by the addition of ascorbic acid, propyl gallate, or butylhydroxytoluene.When stored in glass containers, the solution becomes very pH sensitive; therefore, preparations using sorbic acid as a preservative should be tested for their microbial purity after prolonged periods of storage.
Aqueous solutions of sorbic acid without the addition of antioxidants are rapidly decomposed when stored in polypropylene, polyvinylchloride, and polyethylene containers.
Регуляторный статус
GRAS listed. Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (ophthalmic solutions; oral capsules, solutions, syrups, tablets; topical and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.Sorbic acid запасные части и сырье
сырьё
Sorbic acid поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-29-89586680 +86-15129568250 |
China | 22787 | 58 | |
| +86-13637972665 +86-13637972665 |
China | 81 | 58 | |
| 010-82967028 13552068683 |
China | 12443 | 60 | |
| 021-021-57872719 13761402923 |
China | 310 | 60 | |
| 025-025-58369808 17895012682 |
China | 3502 | 58 | |
| 027-83258456 15392830626 |
China | 206 | 58 | |
| 0371-56060979 13838386843 |
China | 507 | 58 | |
| 135-37016375 13573016375 |
China | 506 | 58 | |
| 13003551299 13003551299 |
China | 7566 | 58 | |
| 029-19991803015 19991803015 |
China | 265 | 58 |