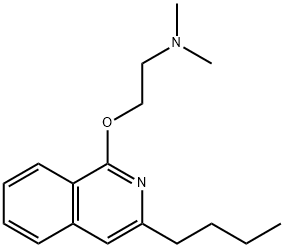
Хинизокаин
- английское имяquinisocaine
- CAS №86-80-6
- CBNumberCB9881536
- ФормулаC17H24N2O
- мольный вес272.39
- EINECS201-700-0
- номер MDLMFCD00242837
- файл Mol86-80-6.mol
химическое свойство
| Температура плавления | 146°C |
| Температура кипения | bp3 155-157° |
| плотность | 0.9848 (rough estimate) |
| показатель преломления | nD20 1.5486 |
| пка | pKa 6.30 (Uncertain) |
| FDA UNII | 772EN3BH6I |
| Код УВД | D04AB05 |
Хинизокаин химические свойства, назначение, производство
Создатель
Quotane,SKF,US,1951Производственный процесс
A mixture of 10.0 grams of β-dimethylaminoethanol and 1.9 grams of sodium in 90 cc of dry xylene was heated at 95°C for 5 hours. To the resulting solution was added at 30°C, 18 grams of 3-butyl-1-chloroisoquinoline. The solution, which turned very dark, was heated at 100°-125°C for 3.5 hours. The mixture was extracted with two 100 cc portions of 2N hydrochloric acid solution. The acid solution was made strongly alkaline with 40% potassium hydroxide solution and the oil which separated was taken into ether. The ether solution was washed with two 100 cc portions of water saturated with sodium chloride, and then dried over anhydrous sodium sulfate for 3 hours. The sodium sulfate was removed by filtration and the ether by distillation. Distillation of the residual oil gave a colorless liquid, BP 155°-157°C/3mm.Терапевтическая функция
Local anestheticХинизокаин запасные части и сырье
сырьё
Хинизокаин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| 029-029-63373950 15353716720 |
China | 10002 | 58 |