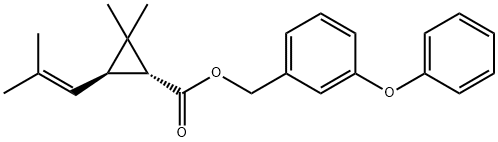
D-Phenothrin
- русский язык имя
- английское имяD-Phenothrin
- CAS №26046-85-5
- CBNumberCB9699395
- ФормулаC23H26O3
- мольный вес350.45
- EINECS247-431-2
- номер MDLMFCD01735786
- файл Mol26046-85-5.mol
химическое свойство
| Температура кипения | 437.0±45.0 °C(Predicted) |
| плотность | 1.120±0.06 g/cm3(Predicted) |
| давление пара | 1.9×10-5Pa (21.4 °C) |
| температура хранения | Sealed in dry,2-8°C |
| растворимость | Chloroform (Slightly), Methanol (Slightly) |
| форма | Oil |
| Растворимость в воде | <0.01 mg l-1 (25 °C) |
| цвет | Colourless to Dark Yellow |
| Справочник по базе данных CAS | 26046-85-5(CAS DataBase Reference) |
| FDA UNII | 6JX527V7HJ |
| Система регистрации веществ EPA | d-Phenothrin (26046-85-5) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P391:Ликвидировать просыпания/проливы/утечки.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
D-Phenothrin химические свойства, назначение, производство
Использование
Phenothrin is used for the control of insects for public health. It is also used to protect stored grain.Метаболический путь
Phenothrin is the name given to the 1RS-cis-trans isomer mixture (racemic). The product now used is d-phenothrin which is 95% 1R and 75% trans. It has no field use because the chrysanthemate moiety is very sensitive to photodegradation. Nevertheless, information on its photochemistry and its fate in soils and plants has been published. Several studies in rodents have been reported; this is a reflection of its use in public health. Phenothrin is degraded mainly by photo-oxidation and by hydrolysis and oxidation in plants and animals.Деградация
Phenothrin is stable under normal storage conditions but it is labile to base, being hydrolysed to trans-2,2-dimethyl-3-(2-methylprop-1-enyl)- cyclopropanecarboxylic acid (11, trans-chrysanthemic acid) and 3-phenoxybenzyl alcohol (13,3PBAlc) (Scheme 2). It is sensitive to light and, for example, as a thin film at midday in the summer at 55 ° N, it was degraded with a DT50 of 2.5-3.0 hours (Samsonov and Makarov, 1996).When (lR)-trans-[14C-carboxyl]phenotwhraisn irradiated in degassed benzene solution, the only product was the cis-isomer. However, in oxygenated benzene solution, degradation was about 10-fold faster and many products were detected (Ruzo et al., 1982). A similar array of products was seen on exposure of thin films to sunlight. Major products (Scheme 1) were formed by oxidation at the isobutylene substituent giving the epoxide (2), the alcohol (3), the aldehyde (4) and the carboxylic acid (5). Caronaldehyde (6) and the caronic acid derivative (7) were formed by cleavage of ozonolysis products and the hydroperoxide (8) was formed by ene reactions at the 1’-position. Ester cleavage to 3PBAlc, 3PBAl and 3PBA (not shown in Scheme 1) was relatively minor.
D-Phenothrin поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-13734021967 +8613734021967 |
China | 1001 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 |
D-Phenothrin Обзор)
1of4