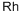
Родий
- английское имяRhodium
- CAS №7440-16-6
- CBNumberCB9365715
- ФормулаRh
- мольный вес102.91
- EINECS231-125-0
- номер MDLMFCD00011201
- файл Mol7440-16-6.mol
| Температура плавления | 1966 °C (lit.) |
| Температура кипения | 3727 °C (lit.) |
| плотность | 12.41 g/cm3 (lit.) |
| температура хранения | Inert atmosphere,Room Temperature |
| растворимость | insoluble in acid solutions, slightly soluble in aqua regia |
| форма | wire |
| Удельный вес | 12.41 |
| цвет | Red |
| РН | 0.5 (20°C in H2O) |
| удельное сопротивление | 4.33 μΩ-cm, 20°C |
| Растворимость в воде | Insoluble |
| Мерк | 14,8186 |
| Пределы воздействия | ACGIH: Ceiling 2 ppm OSHA: Ceiling 5 ppm(7 mg/m3) NIOSH: IDLH 50 ppm; Ceiling 5 ppm(7 mg/m3) |
| Справочник по базе данных CAS | 7440-16-6(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2 |
| FDA UNII | DMK383DSAC |
| Справочник по химии NIST | Rhodium(7440-16-6) |
| Система регистрации веществ EPA | Rhodium (7440-16-6) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | C,Xi,F | |||||||||
| Заявления о рисках | 36/38-11-36/37/38-36-34-23 | |||||||||
| Заявления о безопасности | 26-24/25-16-22-36-17-45-36/37/39 | |||||||||
| РИДАДР | UN 3089 4.1/PG 2 | |||||||||
| OEB | D | |||||||||
| OEL | TWA: 0.1 mg/m3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | VI9069000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 4.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 7110390000 | |||||||||
| Банк данных об опасных веществах | 7440-16-6(Hazardous Substances Data) | |||||||||
| ИДЛА | 100 mg Rh/m3 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
H332:Вредно при вдыхании.
H228:Воспламеняющееся твердое вещество.
H413:Может вызвать долгосрочные отрицательные последствия для водных организмов.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P241:Использовать взрывобезопасное оборудование и освещение.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P314:В случае плохого самочувствия обратиться к врачу.
P403+P233:Хранить в хорошо вентилируемом месте в плотно закрытой/герметичной таре.
P405:Хранить в недоступном для посторонних месте.
Родий химические свойства, назначение, производство
Описание
Rhodium is one of the platinum group elements, and is found at very low concentrations in the Earths crust. Rhodium was discovered by William Hyde Wollaston (England) in 1804. The origin of the name comes from the Greek word rhodon, meaning rose. The plated solid is very corrosion resistant and exceptionally hard. While inert in air and acids, it can produce a violent reaction to chlorine, bromine pentafluoride, bromine trifluoride, and fluorine monoxide.Химические свойства
Rhodium, together with platinum, palladium, iridium, ruthenium, and osmium, is one of the platinum-group metals in Group VIII of the Periodic Table. Rhodium metal is a white, hard, ductile, malleable solid with a bluish-gray luster. soluble in ether, alcohol, and water. The alloys of rhodium can also be used in high temperature conditions (i.e., thermocouples and crucibles). It also can be used in electroplating glass products due to its reflective properties.
Физические свойства
Rhodium is a hard shiny-white metal that resists corrosion from oxygen, moisture, andacids at room temperatures. As a member of group 8 (VIII), 45Rh shares many chemical andphysical properties with cobalt (27Co) just above it and iridium (77Ir) below it in the verticalgroup. Therefore, it is considered one of the elements that are transitory between metals andnonmetals. It is rare and only found in combination with platinum ores.Rhodium’s melting point is 1,966°C, its boiling point is 3,727°C, and its density is 12.41g/cm3.
Изотопы
There are 52 isotopes of rhodium, ranging from Rh-89 to Rh-122. All are producedartificially with relatively short half-lives except one stable isotope, Rh-103, whichconstitutes 100% of the element’s existence in the Earth’s crust.Происхождение имени
Named after the Greek word rhodon, which means “rose,” because of the reddish color of its salt compounds.Вхождение
Rhodium is rare, but not as rare as ruthenium. It makes up only 1 part in 20 million of theelements found in the Earth’s crust. Even so, it is considered the 79th most abundant elementand is found mixed with platinum ore, and to a lesser extent, it is found with copper andnickel ores. It is found in Siberia, South Africa, and Ontario, Canada.Rhodium is recovered from platinum and other ores by refining and purification processesthat start by dissolving the other platinum group metals and related impurities with strongacids that do not affect the rhodium itself. Any remaining platinum group elements areremoved by oxidation and bathing the mixture in chlorine and ammonia.
Rhodium is usually produced as a powder and can be formed by either casting or powdermetallurgy.
Характеристики
Rhodium is one of the six platinum transition elements that include Ru, Rh, Pd, Os, Ir, andPt. Of these metals, rhodium has the highest electrical and thermal conductivity. Although arelatively scarce metal, rhodium makes an excellent electroplated surface that is hard, wearswell, and is permanently bright—ideal for plating the reflectors in automobile headlights.История
Wollaston discovered rhodium in 1803-4 in crude platinum ore he presumably obtained from South America. Rhodium occurs native with other platinum metals in river sands of the Urals and in North and South America. It is also found with other platinum metals in the copper-nickel sulfide ores of the Sudbury, Ontario region. Although the quantity occurring here is very small, the large tonnages of nickel processed make the recovery commercially feasible. The annual world production of rhodium in 1999 was only about 9000 kg. The metal is silvery white and at red heat slowly changes in air to the sesquioxide. At higher temperatures it converts back to the element. Rhodium has a higher melting point and lower density than platinum. Its major use is as an alloying agent to harden platinum and palladium. Such alloys are used for furnace windings, thermocouple elements, bushings for glass fiber production, electrodes for aircraft spark plugs, and laboratory crucibles. It is useful as an electrical contact material as it has a low electrical resistance, a low and stable contact resistance, and is highly resistant to corrosion. Plated rhodium, produced by electroplating or evaporation, is exceptionally hard and is used for optical instruments. It has a high reflectance and is hard and durable. Rhodium is also used for jewelry, for decoration, and as a catalyst. Fifty-two and isomers are now known. Rhodium metal (powder) costs about $180/g (99.9%).isotopesИспользование
Rhodium is a transition metal catalyst used in a multitude of inorganic synthesis.Методы производства
Pure rhodium is prepared by the reduction of its ammonium salt (dichloropentaaminorhodium).Определение
A rare silvery hard transition metal. It is difficult to work and highly resistant to corrosion. Rhodium occurs native but most is obtained from copper and nickel ores. It is used in protective finishes, alloys, and as a catalyst. Symbol: Rh; m.p. 1966°C; b.p. 3730°C; r.d. 12.41 (20°C); p.n. 45; r.a.m. 102.90550.Общее описание
This product has been enhanced for energy efficiency.Опасность
The powder and dust of rhodium metal are flammable in air. Some of the compounds maycause skin irritations. It is best to use approved laboratory procedures when handling any ofthe six elements in the platinum family of metals.Угроза здоровью
There are no data demonstrating acute or chronic rhodium-related diseases; irritation and sensitization have occasionally been reported in humans from exposure to the salts of rhodium. Solutions of insoluble salts splashed in the eye may cause mild irritation.Промышленное использование
Metallic rhodium is the whitest of the platinum metals and does not tarnish under atmospheric conditions. It is insoluble in most acids, including aqua regia, but is attacked by chlorine at elevated temperatures and by hot fuming sulfuric acid. Liquid rhodium dissolves oxygen, and ingots are made by argon-arc melting. At temperatures above 1200 C, rhodium reacts with oxygen to form rhodium oxide, Rh2O3. Rhodium is used to make the nibs of writing pens, to make resistance windings in high-temperature furnaces, for high-temperature thermocouples, as a catalyst, and for laboratory dishes. It is the hardest of the platinum-group metals; the annealed metal has a Brinell hardness of 135. Rhodium is also valued for electroplating jewelry, electric contacts, hospital and surgical instruments, and especially reflectors.The most important alloys of rhodium are rhodium platinum. They form solid solutions in any proportion, but alloys of more than 40% rhodium are rare. Rhodium is not a potent hardener of platinum but increases its high-temperature strength. It is easily workable and does not tarnish or oxidize at high temperatures. These alloys are used for thermocouples and in the glass industry.
Профиль безопасности
Handle carefully. It may be a sensitizer but not to the same extent as platinum. Most rholum compounds have only moderate toxicity by ingestion. Flammable when exposed to heat or flame. Violent reaction with chlorine, bromine pentafluoride, bromine trifluoride, and OF2. A catalytic metalВозможный контакт
Rhodium has few applications by itself, as in rhodium plating of white gold jewelry or plat- ing of electrical parts, such as commutator slip rings, but, mainly, rhodium is used as a component of platinum alloys. Rhodium-containing catalysts have been proposed for use in automotive catalytic converters for exhaust gas cleanup.Канцерогенность
Chick embryos exposed to rhodium on the eighth day of incubation were stunted; mild reduction of limb size and feather growth inhibition were also observed. A number of rhodium compounds have tested positive in bacterial assays for genetic altering capability.Экологическая судьба
The most common route by which rhodium enters the environment is as a component of automobile exhaust resulting from use of catalytic converters. Rhodium is insoluble in water and all acids, with the exception that very finely separated material may be dissolved in concentrated sulfuric acid and aqua regia.Being largely inert, rhodium can undergo long-range transport, and particulate phase matter generally leaves the atmosphere by wet or dry deposition. In an aqueous environment, rhodium can form complexes with halide and nitrogen donor ligands, which may be water soluble, but reactions can be dictated by pH, redox potential, and what material is available for creating ligands. Reactions in soil can depend on these same factors, as well as chloride concentrations, and rhodium is seen to be mobile only in highly acidic soils.
Rhodium has been seen to bioaccumulate in both fresh and salt water species, and has the potential to biomagnify.
Перевозки
Flammable powder, Hazard Class: 4.1; Labels: 4.1-Flammable solid.Несовместимости
Flammable as a dust, fume, or powder may form explosive mixture with air. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanga- nates, perchlorates, chlorine, bromine, fluorine, etc.); con- tact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, bromine pentafluoride, and bromine trifluoride; chlorine trifluoride; oxygen difluoride.Утилизация отходов
Recovery in view of the high economic value. Recovery techniques for recycling of rhodium in plating wastes and spent catalysts have been described in the literature.Родий запасные части и сырье
Родий поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | ||
| +undefined-86-1523780-4566 +undefined15237804566 |
China | 511 | 58 | ||
| +8619931165850 | China | 1000 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | ||
| +86-18532138899 +86-18532138899 |
China | 939 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 |