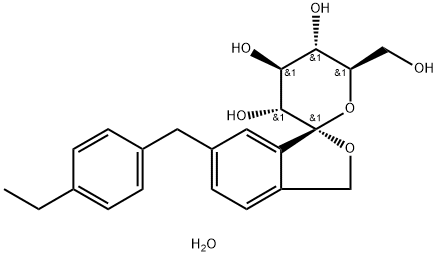
TOFOGLIFLOZIN
- русский язык имя
- английское имяTOFOGLIFLOZIN
- CAS №1201913-82-7
- CBNumberCB92667680
- ФормулаC22H26O6.H2O
- мольный вес404.46
- номер MDLMFCD28385880
- файл Mol1201913-82-7.mol
химическое свойство
| температура хранения | Store at -20°C |
| растворимость | DMF: 30 mg/ml; DMSO: 30 mg/ml; Ethanol: 30 mg/ml; Ethanol:PBS (pH7.2)(1:20): 0.05 mg/ml |
| форма | A crystalline solid |
| цвет | White to off-white |
| FDA UNII | P8DD8KX4O4 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
TOFOGLIFLOZIN химические свойства, назначение, производство
Описание
Tofogliflozin hydrate, which is a sodium-glucose co-transporter 2 inhibitor, was approved in Japan for the treatment of type 2 diabetes at the same time as luseogliflozin hydrate (XIX). The drug was discovered by Chugai Pharmaceutical and jointly developed with Sanofi-Aventis and Kowa. Tofogliflozin hydrate reduces glucose levels by inhibiting the reuptake of glucose by selectively inhibiting SGLT2, and plays a key role in the reuptake of glucose in the proximal tubule of the kidneys.Синтез
Reduction of commercially available 2-bromoterephtalic acid (268) through the use of trimethoxyborane and borane- THF proceeded in 89% yield to afford diol 269. Subjection of this compound to 2-methoxypropene (270) under acidic conditions generated bis-acetonide 271. This bromide then underwent lithium¨Chalogen exchange followed by exposure to magnesium bromide and treatment with lactone 272 (which was prepared by persilylation of commercially available (3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2Hpyran- 2-one (277). This mixture was worked up with aqueous ammonium chloride and upon treatment with p-TsOH in methanol resulted in spiroacetal 273. Next, global protection of all alcohol functionalities within 273 was affected by reaction with methylchloroformate and DMAP in acetonitrile. The benzyl carbonate within 274 was selectively exchanged via Suzuki coupling with 4-ethylphenylboronic acid (275) to afford methylene dibenzyl system 276. Subsequent treatment with aqueous sodium hydroxide in methanol followed by crystallization from 1:6 acetone and water furnished the desired product tofogliflozin hydrate (XXXIV) in 75% yield.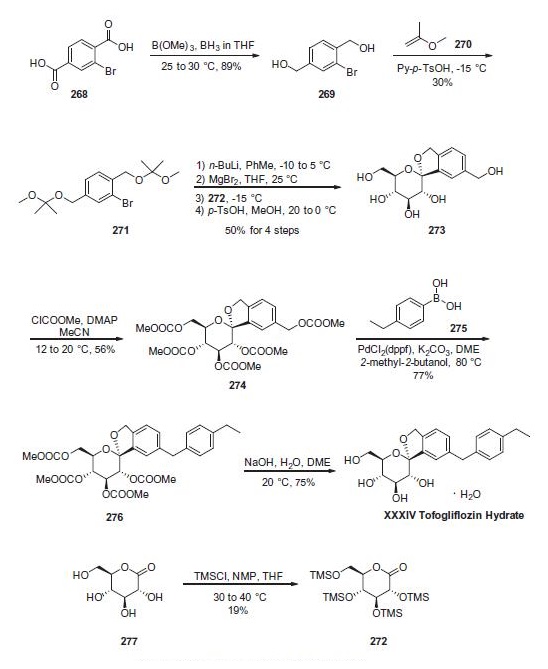
TOFOGLIFLOZIN поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 15380796838 | CHINA | 340 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +1-631-485-4226 | United States | 19553 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| 86-571-88216897,88216896 13588875226 |
CHINA | 6312 | 58 | |
| +8618330912755 | China | 1658 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| China | 12341 | 58 |