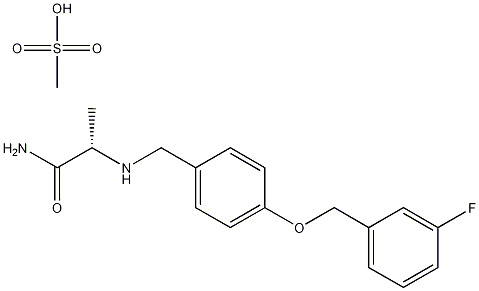
Safinamide mesylate salt
- английское имяSafinamide mesylate
- CAS №202825-46-5
- CBNumberCB92510036
- ФормулаC17H19FN2O2.CH4O3S
- мольный вес398.4490232
- номер MDLMFCD15145475
- файл Mol202825-46-5.mol
| Температура плавления | 210° (dec) |
| альфа | D25 +12.9° (c = 1.1% in 98% acetic acid) |
| температура хранения | 2-8°C |
| растворимость | H2O: ≥15mg/mL |
| форма | powder |
| цвет | white to tan |
| оптическая активность | [α]/D +9.5 to +14°, c = 1 (95% acetic acid) |
| Растворимость в воде | H2O: ≥15mg/mL |
| InChI | InChI=1/C17H19FN2O2.CH4O3S/c1-12(17(19)21)20-10-13-5-7-16(8-6-13)22-11-14-3-2-4-15(18)9-14;1-5(2,3)4/h2-9,12,20H,10-11H2,1H3,(H2,19,21);1H3,(H,2,3,4)/t12-;/s3 |
| ИнЧИКей | YKOCHIUQOBQIAC-ZLBVLXFENA-N |
| SMILES | CS(O)(=O)=O.C(N)(=O)[C@@H](NCC1=CC=C(OCC2=CC=CC(F)=C2)C=C1)C |&1:8,r| |
| FDA UNII | YS90V3DTX0 |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H302:Вредно при проглатывании.
H361d:Предполагается, что данное вещество может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Safinamide mesylate salt химические свойства, назначение, производство
Описание
Safinamide methanesulfonate was approved in February 2015 by the EMA for the treatment of mid- to late-stage fluctuating Parkinson’s disease. This approval included use of the drug as an add-on therapy for use with levodopa, either alone or in combination with other existing therapies for Parkinson’s disease.51 Safinamide methanesulfonate, an oral α-aminoamide originally discovered by Farmitalia Carlo Erba and later developed by Newron/Zambon, functions as a highly selective and reversible inhibitor of MAO-B, leading to increased levels of dopamine and subsequent improvement in the motor symptoms of Parkinson’s disease, side effects that often result from use of other traditional treatments relying on dopamine replacement therapy.Химические свойства
Safinamide mesylate is a white to off-white crystalline powder. Safinamide mesylate is freely soluble in water, methanol and dimethyl sulfoxide. Safinamide mesylate is sparingly soluble in ethanol and is practically insoluble in ethyl acetate. In aqueous buffers that span a pH range of 1.2 to 7.5, safinamide mesylate is highly soluble at pH 1.2 and 4.5 but shows low solubility (<0.4 mg/mL) at pH 6.8 and 7.5.Использование
Safinamide mesylate salt has been used as a reference drug to study its inhibitory effect on human monoamine oxidases (hMAO-A and hMAO-B).Механизм действия
Safinamide employs several mechanisms of action, functioning as both a dopaminergic agent through inhibition of MAO-B as well as a nondopaminergic agent via selective calcium and sodium channel modulation, leading to inhibition of glutamate release. At least one of several clinical studies of patients with mid- to late-stage Parkinson’s disease showed increased daily ON time (periods of symptom control) without accompanying motor complications (dyskinesias) upon treatment with safinamide, while studies of early stage Parkinson’s disease patients treated with this drug showed significantly improved motor symptoms during the 18-month study. Additionally, safinamide is chemically and metabolically stable, is well tolerated in patients, and has not exhibited serious adverse effects even upon treatment at higher dosage ranges.Побочные эффекты
Common adverse events in clinical trials (in more than 1% of people) included nausea, dizziness, tiredness, sleeplessness, or thostatic hypotension (low blood pressure) and headache. There was no significant difference in the occurrence of these effects between safinamide and placebo.Safinamide mesylate salt поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +16313385890 | United States | 3868 | 58 | ||
| +86-0086-531-88259693 +86-18660188356 |
China | 78 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-18600796368 +86-18600796368 |
China | 484 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| 010-60279497 | CHINA | 1803 | 55 | ||
| 008657128800458; +8615858145714 |
China | 7738 | 55 | ||
| 025-83697070 | CHINA | 3009 | 60 | ||
| +undefined-21-51877795 | China | 32965 | 60 |