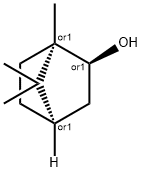
Борнеол
- английское имяBorneol
- CAS №507-70-0
- CBNumberCB8697516
- ФормулаC10H18O
- мольный вес154.25
- EINECS208-080-0
- номер MDLMFCD00003759
- файл Mol507-70-0.mol
| Температура плавления | 206-209 °C |
| Температура кипения | 210 °C(lit.) |
| альфа | -0.5~+0.5゜(20℃/D)(c=5,C2H5OH) |
| плотность | 1.011 |
| плотность пара | 5.31 (vs air) |
| давление пара | 33.5 mm Hg ( 25 °C) |
| FEMA | 2157 | BORNEOL |
| показатель преломления | 1.4831 (estimate) |
| Fp | 150 °F |
| растворимость | DMSO:30.0(Max Conc. mg/mL);194.49(Max Conc. mM) Ethanol:30.0(Max Conc. mg/mL);194.49(Max Conc. mM) |
| форма | powder to crystal |
| пка | 15.36±0.60(Predicted) |
| цвет | White to Almost white |
| Запах | at 10.00 % in dipropylene glycol. pine woody camphor balsamic |
| Odor Type | balsamic |
| Растворимость в воде | insoluble |
| Мерк | 14,1338 |
| Номер JECFA | 1385 |
| Стабильность | Stable. Highly flammable. Incompatible with strong oxidizing agents. |
| ИнЧИКей | DTGKSKDOIYIVQL-WEDXCCLWSA-N |
| LogP | 3.6 at 20℃ |
| Справочник по базе данных CAS | 507-70-0(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | BORNEOL |
| FDA UNII | M89NIB437X |
| Справочник по химии NIST | Borneol(507-70-0) |
| Система регистрации веществ EPA | Borneol (507-70-0) |
| Коды опасности | F,Xi,Xn | |||||||||
| Заявления о рисках | 11-43-22 | |||||||||
| Заявления о безопасности | 16-36/37 | |||||||||
| РИДАДР | UN 1312 4.1/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | DT5095000 | |||||||||
| Класс опасности | 4.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29061990 | |||||||||
| Банк данных об опасных веществах | 507-70-0(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
H228:Воспламеняющееся твердое вещество.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P241:Использовать взрывобезопасное оборудование и освещение.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312+P330:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии. Прополоскать рот.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Борнеол химические свойства, назначение, производство
Описание
l-Borneolum was originally from the Compositae Aenean Blumea balsamifera (L.) DC (Ai Na Xiang). Its crystals were made after being extracted from the fresh leaves of Ai Na Xiang. It is an optical isomer with natural borneol. It’s usually used for making spice the same as natural borneol, synthesized borneol, and so on .Ai Na Xiang is a traditional Chinese medicine alias as Da Feng Ai, Niu Er Ai, etc. It has been recorded firstly in Guang Zhi, “Ai Na Xiang came from the west country and looks like the artemisia.” It has been recorded in many Chinese materia medica books, such as Hai Yao Ben Cao, Ling Nan Cai Yaolu, etc. In Zhong hua Ben cao (Chinese Materia Medica), the literature described it as “Ai Na Xiang (l-borneolum) has the fragrance because it is like the artemisia. It can also be prepared to the borneol, so it is called “Bing Pian Ai.” It is believed that this is the earliest records that Ai Na Xiang was made into borneol . In China, the authentic product area of l-borneolum is in Luodian, Guizhou Province . There are no other plant resources reported for producing l-borneolum in China.
Химические свойства
Borneol is a bicyclic terpene alcohol. Borneol is an endo isomer; the corresponding exo isomer is isoborneol:Borneol occurs abundantly in nature as a single enantiomer or, less frequently, as the racemate. (1S,2R,4S)-(?)-Borneol occurs particularly in oils from Pinaceae species and in citronella oil. (1R,2S,4R)-(+)-Borneol is found, for example, in camphor oil (Ho-Sho oil), rosemary, lavender, and olibanum oils. Borneol is a colorless, crystalline solid. (+)-Borneol has a camphoraceous odor, with a slightly sharp, earthy, peppery note, which is less evident in (?)-borneol. Commercial borneol is often levorotatory ([α]20 D ?18 to ?28 in ethanol) and contains (?)-borneol and up to 40% isoborneol.
Borneol is oxidized to camphor with chromic or nitric acid; dehydration with dilute acids yields camphene. Borneol is readily esterified with acids, but on an industrial scale, bornyl esters are prepared by other routes. For example, levorotatory borneol is synthesized industrially from levorotatory pinenes by Wagner–Meerwein rearrangement with dilute acid, followed by hydrolysis of the resulting esters.
Borneol is used in the reconstitution of the essential oils in which it occurs naturally.
Физические свойства
Appearance: white translucent flakes, bulk or granular crystals. With a clear aroma, spicy, cool, volatile, black smoke, and yellow flame when lit without residue left. Melting point: 201–205? °C.? Soluble in ethanol, chloroform, or ether, almost insoluble in water. Specific optical rotation: ?36.5° to ?38.5° in 5% (g/mL) ethanol solution, stable under sealed condition at room temperature.Pungent, bitter, slightly cold; heart, spleen, and lung meridians entered. (In Chinese traditional medicine’s opinion)
Вхождение
Unlike isoborneol, free or esterified borneol has been identified in more than 250 distillates from plants, herbs, leaves or bark; Compositae, Ericaceae, Lauraceae, Labiatea, Rutaceae; natural borneol can be d or l rotatory, but very seldom also racemic; most frequently encountered is the l-borneol characteristic of Compositae, Graminaceae and almost all Pinaceae; d-borneol characteristic of Cupressaceae, Zingiberaceae, lavender, lavandin and spike oils. Reported found in citrus peel oils (orange, lemon, lime), cinnamon leaf, cassia leaf, ginger, coriander seed, laurel, Ocimum basilicum, Thymus vulgaris L. and Curcuma aeruginosa Roxb.Использование
Perfumery, esters.Определение
ChEBI: A bornane monoterpenoid that is 1,7,7-trimethylbicyclo[2.2.1]heptane substituted by a hydroxy group at position 2.Показания
Sore throat, aphthous, red eyes, purulent ear discharge, convulsions, febrile delirium, sudden faint due to qi depression, stroke, and comaОбщее описание
A white colored lump-solid with a sharp camphor-like odor. Burns readily. Slightly denser than water and insoluble in water. Used to make perfumes.Реакции воздуха и воды
Flammable. Insoluble in water.Профиль реактивности
Borneol is an alcohol. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides.Опасность
Fire risk in presence of open flame.Угроза здоровью
Fire may produce irritating and/or toxic gases. Contact may cause burns to skin and eyes. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.Пожароопасность
Flammable/combustible material. May be ignited by friction, heat, sparks or flames. Some may burn rapidly with flare burning effect. Powders, dusts, shavings, borings, turnings or cuttings may explode or burn with explosive violence. Substance may be transported in a molten form at a temperature that may be above its flash point. May re-ignite after fire is extinguished.Фармаколо?гия
The main effects of l-borneolum are to induce resuscitation (with aromatic stimulation), clear stagnated fire (fever feeling), remove nebula to improve eyesight, and relieve swelling and pain. In traditional Chinese medicine, borneol is often used as an envoy drug and combined with other drugs.The modern pharmacological researches showed that l-borneolum can cross the blood-brain barrier (BBB) through increasing cell membrane fluidity, Na+ -K+- ATPase activity, decreasing membrane potential, and regulating intracellular calcium concentration, which is involved in the effect of resuscitation .
The key brain-protective mechanism of l-borneolum and synthetic borneol is closely related to the regulation of P-glycoprotein pathway, lipid peroxidation, and nitric oxide pathway. In addition, it can regulate the calcium pathway, which is the main biological mechanism of “Xin-floating-heart” medicinal property of borneol and resuscitation. Based on the statistical results of strength integral law, it demonstrated that the brain-protective effect of l-borneolum is stronger than synthetic borneol, suggesting that we should prefer l-borneolum for the treatment of cerebrovascular disease .
Клиническое использование
l-Borneolum is used as a kind of borneol in clinical usage all along, but its clinical usage is fewer than borneol considering its clinical efficacy is weaker than borneol. The main reasons may be the following aspects: the discovery of l-borneolum is later than borneol, and the processing technology of l-borneolum is far behind borneol which leads to high impurities. l-Borneolum has less adverse reactions the same as borneol, including gastrointestinal irritation, reproductive toxicity, and allergic reactions.Борнеол запасные части и сырье
Борнеол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 028-87075086 13350802083 |
CHINA | 1824 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| +86-28-82633860; +8618080483897 |
China | 3772 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 |