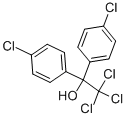
1,1-Бис(п-хлорфенил)-2,2,2-трихлорэтанол
- английское имяDicofol
- CAS №115-32-2
- CBNumberCB8424167
- ФормулаC14H9Cl5O
- мольный вес370.49
- EINECS204-082-0
- номер MDLMFCD00055271
- файл Mol115-32-2.mol
| Температура плавления | 78.5°C |
| Температура кипения | 225°C |
| плотность | 1.45 |
| давление пара | 5.3 x 10-5 Pa |
| показатель преломления | 1.6000 (estimate) |
| Fp | 11 °C |
| температура хранения | APPROX 4°C |
| растворимость | DMSO: Slightly soluble |
| форма | A solid |
| пка | 10.70±0.29(Predicted) |
| Растворимость в воде | Slightly soluble. <0.01 g/100 mL at 22 ºC |
| Мерк | 13,3113 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. Hydrolyzes in basic solution. Corrodes some metals. |
| Справочник по базе данных CAS | 115-32-2(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 5 |
| FDA UNII | W4WMM0WS91 |
| МАИР | 3 (Vol. 30, Sup 7) 1987 |
| Справочник по химии NIST | Acetic acid, [3,5-diiodo-4-(4-hydroxy-3-iodophenoxy) phenyl]-, 2-[diethylamino]ethyl ester, hydrochloride(115-32-2) |
| Система регистрации веществ EPA | Dicofol (115-32-2) |
| Пестициды Закон о свободе информации (FOIA) | Dicofol |
| Коды опасности | Xn;N,N,Xn,T,F | |||||||||
| Заявления о рисках | 21/22-38-43-50/53-39/23/24/25-23/24/25-11 | |||||||||
| Заявления о безопасности | 36/37-60-61-45-16-7 | |||||||||
| РИДАДР | UN 2761/3077 | |||||||||
| RTECS | DC8400000 | |||||||||
| Банк данных об опасных веществах | 115-32-2(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 in rats (mg/kg): 1495 orally; 1150 i.p. (Brown) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H302+H312:Вредно при проглатывании или при попадании на кожу.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
1,1-Бис(п-хлорфенил)-2,2,2-трихлорэтанол химические свойства, назначение, производство
Химические свойства
Pure dicofol is available as a white or grey powder or colourless crystal solid, while the technical dicofol is a red-brown or amber viscous liquid with an odour like fresh-cut hay. Dicofol undergoes decomposition on burning or on contact with acids, acid fumes, or bases producing toxic and corrosive fumes including hydrogen chloride.Dicofol is combustible and incompatible with strong oxidising agents. Dicofol is soluble in most aliphatic and aromatic solvents and most common organic solvents but practically insoluble in water and hydrolyses in basic solution.
Использование
Acaricide, organochlorine pesticide. Dicofol is a non-systemic acaricide/miticide currently registered in the US and Canada for use on a wide variety of crops.Определение
ChEBI: A tertiary alcohol that is DDT in which the benzylic hydrogen has been replaced by a hydroxy group.Общее описание
Dicofol or kelthane is a white crystalline, wettable powder dissolved in a liquid carrier, (water). The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Since Dicofol is a liquid Dicofol can easily penetrate the soil and contaminate groundwater and nearby streams. Dicofol can cause illness by inhalation, skin absorption, and/or ingestion. Dicofol is used as a pesticide.Реакции воздуха и воды
Hydrolyzed in alkaline media to dichlorobenzophenone and chloroform. Insoluble in water.Профиль реактивности
Dicofol is an organochlorine bridged diphenyl. Halogenated aliphatic compounds are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Halogenated aliphatics are incompatible with strong oxidizing and reducing agents. Also, they are incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. Dicofol hydrolyzes in alkali. Dicofol is slightly corrosive to metals. Contact with steel at elevated temperatures causes formation of toxic gases. .Угроза здоровью
Exposures to dicofol cause adverse health effects and poisoning. Occupational workers suffer harmful effects on inhalation, ingestion and through skin contact. The symptoms of poisoning include, but are not limited to, nausea, dizziness, weakness, and vomiting from ingestion or respiratory exposure, skin irritation or rash from dermal exposure. Dicofol-poisoned occupational workers show skin sensitization, conjunctivitis of the eyes, and pathomorphological changes in the liver, kidneys, and CNS. After exposures to high concentrations of dicofol, workers show nervousness, hyperactivity, headache, nausea, vomiting, unusual sensations, fatigue, convulsions, coma, respiratory failure, and death. However, published literature is limited and more data is required on occupational workers as well as the general population.Сельскохозяйственное использование
Insecticide, Acaricide: Not approved for use in EU countries. Severely Restricted for use in EU (containing>78% p,p'-dicofol or 1 g/kg of DDT and DDT-related compounds). Dicofol is an organochlorine miticide/pesticide used for foliar applications, mostly on cotton, apples, and citrus crops. Other crops include: strawberries, mint, beans, peppers, tomatoes, pecans, walnuts, stonefruit, cucurbits, and nonresidential lawns/ornamentals. Formulations registered for use on food/feed crops include emulsifiable concentrates, and wettable powder formulations. These formulations may be applied as concentrated or dilute sprays using aircraft, duster, groundboom, and sprayer. Dicofol is manufactured from DDT. In 1986, use of dicofol was temporarily canceled by the U.S. EPA because of concerns raised by high levels of DDT contamination. However, it was reinstated when it was shown that modern manufacturing processes can produce technical-grade dicofol which contains <0.1% DDT.Торговое название
ACARIN®[C]; CALLIFOL®; CARBAX®; CEKUDIFOL®; DECOFOL®; DICOMITE®; DIFOL®; FERRIAMICIDE®; FUMITE DICOFOL®; FW 293®; HIFOL®; KELTANE®; KELTHANE®; P,P'- KELTHANE®; KELTHANETHANOL®; MILBOL®; MITIGAN®; TIKTOK®; VAPCOTHION®, dicofolВозможный контакт
A potential danger to those involved in manufacture, formulation and application of this organochlorine pesticide. Used as acaricide (miticide) in agricultural and nonagricultural applications. Similar in structure to DDT.Экологическая судьба
Plant. The major metabolite reported on apples is 4,4′-dichlorobenzophenone. On apples, dicofol concentrations decreased from 702 ppm to 436 ppm after 15 days. 4,4′Dichlorobenzophenone increased from 25.5 ppm at time of spraying to 25.5 ppm 15 days after spraying (Archer, 1974). Four days after spraying cucumbers with dicofol, residues decreased from 0.95–1.6 ppm to 0.4–1.5 ppm. No residues were detected 8 days after spraying (Nazer and Masoud, 1986). A half-life of 6 days was reported for dicofol in alfalfa (Akesson and Yates, 1964).Chemical/Physical. When dicofol was exposed to sunlight for 20 days, a 10% yield of 4,4′-dichlorobenzophenone was obtained. Solvents containing dicofol and exposed to UV light resulted in the formation of chlorobenzilic acid esters (Vaidyanathasw
Метаболический путь
Dicofol(1) is an analogue of DDT. However, the replacement of a hydrogen atom at position 1 by a hydroxyl group results in a fundamental change in chemical properties and increases the lability of the molecule. It breaks readily down to 4,4’-dichlorobenzophenone (2) thermally or on hydrolysis and this compound is a major metabolite in mammals and plants.Перевозки
UN2996 Organochlorine pesticides, liquid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3082 Environmentally hazardous substances, liquid, n.o. s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Несовместимости
Incompatible with alkaline pesticides, strong acids; acid fumes; aliphatic amines; isocyanates. Halogenated aliphatic compounds are moderately or very reactive. Halogenated organics generally become less reactive as more of their hydrogen atoms are replaced with halogen atoms. Halogenated aliphatics are incompatible with strong oxidizing and reducing agents. Also, they are incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. Dicofol hydrolyzes in alkali. It is slightly corrosive to metals. Contact with steel at elevated temperatures causes formation of toxic gasesУтилизация отходов
In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.