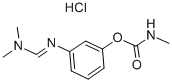
FORMETANATE HYDROCHLORIDE
- русский язык имя
- английское имяFORMETANATE HYDROCHLORIDE
- CAS №23422-53-9
- CBNumberCB8372321
- ФормулаC11H16ClN3O2
- мольный вес257.72
- EINECS245-656-0
- номер MDLMFCD00055560
- файл Mol23422-53-9.mol
| Температура плавления | 201℃ (decomposition) |
| плотность | 1.3616 (rough estimate) |
| давление пара | 1.6 x 10-6 Pa (25 °C) |
| показатель преломления | 1.5270 (estimate) |
| температура хранения | 0-6°C |
| растворимость | DMSO (Slightly), Methanol (Slightly), Water (Slightly) |
| пка | 8.0 (base) |
| Растворимость в воде | 822,000 mg l-1 (25 °C) |
| Стабильность | Hygroscopic |
| Рейтинг продуктов питания EWG | 1-3 |
| FDA UNII | W0Y3OP0N2Z |
| Система регистрации веществ EPA | Formetanate hydrochloride (23422-53-9) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | T+,N |
| Заявления о рисках | 26/28-43-50/53 |
| Заявления о безопасности | 24-28-37/39-45-60-61 |
| РИДАДР | 2757 |
| WGK Германия | 3 |
| RTECS | FC2514000 |
| Класс опасности | 6.1(a) |
| Группа упаковки | II |
| кода HS | 29252900 |
| Банк данных об опасных веществах | 23422-53-9(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H300+H330:Смертельно при проглатывании или при вдыхании.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310+P330:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
FORMETANATE HYDROCHLORIDE химические свойства, назначение, производство
Химические свойства
Formetanate hydrochloride is a white or yellowish, crystalline solid or powder with a faint odor.Использование
Formetanate is an insecticide and acaricide with contact and stomach action. It is used to control spider mites and some insects (Diptera, Hemiptera and Thysanoptera) on ornamentals, fruit, vegetables and alfalfa.Общее описание
White powder with a faint odor. Used as a plant insecticide, acaricide, and miticide.Реакции воздуха и воды
Hydrolyzed at pH less than 4 [EPA, 1998].Профиль реактивности
FORMETANATE HYDROCHLORIDE is a carbamate ester. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates.Угроза здоровью
Extremely toxic to humans. Not absorbed through contact with skin. Inhalation or ingestion may cause poisoning. Inhibits cholinesterase activity so effects are in relation to action on nervous system and can result in death.Пожароопасность
FORMETANATE HYDROCHLORIDE may burn but does not ignite readily. Container may explode in heat of fire. Hydrolyzed at pH less than 4Сельскохозяйственное использование
Insecticide, Acaricide: Registered for use in the U.S. Not listed for use in EU countries. An insecticide used for thrips and true bug control on fruit crops.Торговое название
CARZOL®; CARZOL® SP; DICARZOL®; ENT 27566®; EP-332®; MORTON® EP332; NOR-AM® EP 332; SCHERING® 36056; SN 36056®Профиль безопасности
Poison by ingestion and intraperitoneal routes. Mddly toxic by skin contact. When heated to decomposition it emits very toxic fumes of NOx and HCl.Возможный контакт
A potential danger to those involved in the manufacture, formulation, and application of this plant insecticide, acaricide and miticide.Метаболический путь
Formetanate is hydrolysed rapidly in field and laboratory soils under aerobic and anaerobic conditions. It is metabolised by similar pathways in plants and animals. It is hydrolytically deaminated to produce a formyl derivative and subsequently hydrolysed at the carbamate ester to give a phenolic product. The phenol is deformylated to 3-aminophenol and acetylated to 3-acetamidophenol. Formetanate and most phase 1 metabolites are found conjugated. Its metabolism has been reviewed by Cool and Jankowski (1985), Fukuto (1972) and Kuhr and Dorough (1976).Перевозки
UN2757 Carbamate pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.Несовместимости
Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrideds and active metals. Contact with active metals or nitrides form flammable gas eous hydrogen. Incompatible with strongly oxidizing acids, peroxides, and hydroperoxides.Утилизация отходов
In accordance with 40CFR 165 recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by follow ing package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥kg/mo) must conform with EPA regulations gov erning storage, transportation, treatment, and waste disposal.