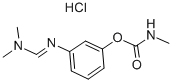
General Description
White powder with a faint odor. Used as a plant insecticide, acaricide, and miticide.
Air & Water Reactions
Hydrolyzed at pH less than 4 [EPA, 1998].
Reactivity Profile
FORMETANATE HYDROCHLORIDE is a carbamate ester. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates.
Health Hazard
Extremely toxic to humans. Not absorbed through contact with skin. Inhalation or ingestion may cause poisoning. Inhibits cholinesterase activity so effects are in relation to action on nervous system and can result in death.
Fire Hazard
FORMETANATE HYDROCHLORIDE may burn but does not ignite readily. Container may explode in heat of fire. Hydrolyzed at pH less than 4
Potential Exposure
A potential danger to those involved
in the manufacture, formulation, and application of this
plant insecticide, acaricide and miticide.
First aid
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, includ ing resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medi cal attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit.
Shipping
UN2757 Carbamate pesticides, solid, toxic,
Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1;
Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Carbamates are incompatible with strong
acids and bases, and especially incompatible with strong
reducing agents such as hydrideds and active metals.
Contact with active metals or nitrides form flammable gas eous hydrogen. Incompatible with strongly oxidizing acids,
peroxides, and hydroperoxides.
Description
Formetanate hydrochloride is a white oryellowish, crystalline solid or powder with a faint odor.Molecular weight=257.75; Freezing/Melting point 5200 C-202 C (decomposes). Hazard Identification (basedon NFPA-704 M Rating System): Health 3, Flammability 1,Reactivity 0. Highly soluble in water.
Chemical Properties
Formetanate hydrochloride is a white or
yellowish, crystalline solid or powder with a faint odor.
Waste Disposal
In accordance with 40CFR
165 recommendations for the disposal of pesticides and
pesticide containers. Must be disposed properly by follow ing package label directions or by contacting your local or
federal environmental control agency, or by contacting
your regional EPA office. Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥kg/mo) must conform with EPA regulations gov erning storage, transportation, treatment, and waste
disposal.
Uses
Formetanate is an insecticide and acaricide with contact and
stomach action. It is used to control spider mites and some insects
(Diptera, Hemiptera and Thysanoptera) on ornamentals, fruit, vegetables
and alfalfa.
Agricultural Uses
Insecticide, Acaricide: Registered for use in the U.S. Not listed for use in
EU countries. An insecticide used for thrips and true
bug control on fruit crops.
Trade name
CARZOL®; CARZOL® SP;
DICARZOL®; ENT 27566®; EP-332®; MORTON®
EP332; NOR-AM® EP 332; SCHERING® 36056; SN
36056®
Metabolic pathway
Formetanate is hydrolysed rapidly in field and laboratory soils under
aerobic and anaerobic conditions. It is metabolised by similar pathways in
plants and animals. It is hydrolytically deaminated to produce a formyl
derivative and subsequently hydrolysed at the carbamate ester to give a
phenolic product. The phenol is deformylated to 3-aminophenol and
acetylated to 3-acetamidophenol. Formetanate and most phase 1 metabolites
are found conjugated. Its metabolism has been reviewed by Cool
and Jankowski (1985), Fukuto (1972) and Kuhr and Dorough (1976).
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling and storage. Store in tightly closed containers in a cool, wellventilated area.
Degradation
Formetanate is hydrolysed rapidly under basic conditions. DT50 values at
pH 5, 7 and 9 (22 °C) are reported to be 65 days, 23 hours and 2 hours,
respectively (PM). The photolysis of formetanate hydrochloride in solutions
of distilled water at pH 3.1 and 7.1 or natural river water was
studied. Samples were irradiated for 4 days by a high pressure Hg
lamp immersed in the solutions. Light of wavelength <286 nm was filtered
out. Analysis of solutions was by TLC, IR and MS. The carbamate moiety
was more stable than the formamidine. Photolysis gave four products,
viz. the formaminophenol (2), the 3-aminophenyl carbamate (3), the
formamino compound (4) and 3-hydroxyphenyl methylcarbamate (5), a product of oxidative deamination of formetanate. Yields increased in the
order given. The proposed pathways of photolysis of formetanate are
given in Scheme 1 (Su and Zabick, 1972). Soil surface photolysis occurs
with a DT50 value of 16 hours (PM).