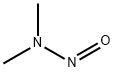
N-метил-N-нитрозометанамин
- английское имяN-NITROSODIMETHYLAMINE
- CAS №62-75-9
- CBNumberCB8364791
- ФормулаC2H6N2O
- мольный вес74.08
- EINECS200-549-8
- номер MDLMFCD00002053
- файл Mol62-75-9.mol
| Температура плавления | <25 °C |
| Температура кипения | 153 °C774 mm Hg(lit.) |
| плотность | 1.01 g/mL(lit.) |
| давление пара | 5 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 142 °F |
| температура хранения | 2-8°C |
| растворимость | Soluble in solvents (U.S. EPA, 1985), including ethanol and ether (Weast, 1986) |
| пка | -3.63±0.70(Predicted) |
| форма | Yellow liquid |
| цвет | Colourless to Light Yellow |
| Растворимость в воде | Miscible (Mirvish et al., 1976) |
| Мерк | 13,6671 |
| констант закона Генри | 0.143 at 25 °C (estimated using a solubility of 1,000 g/L) |
| Диэлектрическая постоянная | 54.0(20℃) |
| Стабильность | Stability Stable, but light-sensitive. Combustible. Incompatible with strong oxidizing agents, strong bases, strong reducing agents. |
| Справочник по базе данных CAS | 62-75-9(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 7-10 |
| FDA UNII | M43H21IO8R |
| Предложение 65 Список | n-Nitrosodimethylamine |
| МАИР | 2A (Vol. 17, Sup 7) 1987 |
| Система регистрации веществ EPA | N-Nitrosodimethylamine (62-75-9) |
| UNSPSC Code | 77101502 |
| NACRES | NA.24 |
| Коды опасности | T+,N,T,F |
| Заявления о рисках | 45-25-26-48/25-51/53-39/23/24/25-23/24/25-11 |
| Заявления о безопасности | 53-45-61-36/37-16 |
| РИДАДР | UN 3382 6.1/PG 1 |
| WGK Германия | 3 |
| RTECS | IQ0525000 |
| Класс опасности | 6.1(b) |
| Группа упаковки | III |
| Банк данных об опасных веществах | 62-75-9(Hazardous Substances Data) |
| Токсичность | LD50 i.p. in rats: 34 mg/kg (Heath) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H350:Может вызывать раковые заболевания.
H372:Поражает органы в результате многократного или продолжительного воздействия.
H411:Токсично для водных организмов с долгосрочными последствиями.
H330:Смертельно при вдыхании.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P284:Использовать средства защиты органовдыхания.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P310:Немедленно обратиться за медицинской помощью.
P314:В случае плохого самочувствия обратиться к врачу.
P403+P233:Хранить в хорошо вентилируемом месте в плотно закрытой/герметичной таре.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
N-метил-N-нитрозометанамин химические свойства, назначение, производство
Химические свойства
N-Nitrosodimethylamine is a yellow oily liquid. Faint, characteristic odor.Физические свойства
Yellow, oily liquid with a faint, characteristic odorИспользование
N-Nitrosodimethylamine is a highly toxic semi-volatile organic compound and a suspected human carcinogen. It induces liver tumors in rats after chronic exposure to low doses (1,2). Drinking water contaminant candidate list 3 (CCL 3) compound as per United States Environmental Protection Agency (EPA). Environmental contaminants; Food contaminants.NDMA and NDEA were found as an impurity in generic versions of valsartan, losartan and irbesartan.Методы производства
Dimethylnitrosamine (DMN) is prepared by the addition of acetic acid and sodium nitrite to dimethylamine. It came into industrial prominence in the manufacture of 1,1- dimethylhydrazine, a rocket fuel component, probably in the 1940s in Germany and in the mid-1950s in the United States. DMN is no longer used except for research. OSHA identified DMN as a carcinogen in 1974. In 1976, the last plant to make DMN was closed. DMN was first considered by IARC in 1971. The current IARC classification of DMN is 2A: “The agent is probably carcinogenic to humans, based upon positive cancer findings in animal studies.” For this reason, DMN is discussed here as an example of one of a large group of nitrosamines generally regarded as carcinogenic. IARC Monographs 1 and 17 and other publications discuss the toxicology, biochemistry, environmental impact, and carcinogenicity of many nitrosamines.Общее описание
Yellow oily liquid with a faint characteristic odor. Boiling point 151-153°C. Can reasonably be expected to be a carcinogen. Used as an antioxidant, as an additive for lubricants and as a softener of copolymers. An intermediate in 1,1-dimethylhydrazine production.Реакции воздуха и воды
Water soluble.Профиль реактивности
N-NITROSODIMETHYLAMINE is sensitive to exposure to light, especially ultraviolet light. Is stable at room temperature for more than 14 days in aqueous solution at neutral and alkaline pH in the absence of light. Slightly less stable at strongly acid pH at room temperature. Incompatible with strong oxidizing agents. Also incompatible with strong bases. Can be reduced by reducing agents. Incompatible with hydrogen bromide in acetic acid. Also photo chemically reactive.Угроза здоровью
N-nitrosodimethylamine (DMN) is a liver toxin and is carcinogenic in many species of test animals.Пожароопасность
When heated to decomposition, N-NITROSODIMETHYLAMINE emits toxic fumes of nitrogen oxides. Avoid exposure to ultraviolet light.Возможный контакт
Nitrosodimethylamine was formerly used in the production of rocket fuels. Presently used as an antioxidant; as an additive for lubricants and as a softener of copolymers. It is used as an intermediate for 1,1-dimethylhydrazine.Канцерогенность
N-Nitrosodimethylamine is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.Перевозки
UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. PG I.Методы очистки
Dry the nitrosamine over anhydrous K2CO3 or dissolve it in Et2O, dry it over solid KOH, filter, evaporate Et2O and distil the yellow oily residue through a 30cm fractionating column discarding the first fraction which may contain Me2N. Also dry over CaCl2 and distil it at atmospheric pressure. All operations should be done in an efficient fume cupboard as the vapors are TOXIC and CARCINOGENIC. [Fischer Chem Ber 8 1588 1875, Romberg Recl Trav Chim, Pays-Bas 5 248 1886, Hatt Org Synth Coll Vol II 211 1961, Krebs & Mandt Chem Ber 108 1130 1975.] 2,6-Dimethyl-2,4,6-octatriene see neo-alloocimene below.Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong acids, especially peracids strong bases. Sensitive to UV light. Should be stored in dark bottles.Утилизация отходов
Pour over soda ash, neutralize with HCl, then flush to drain with large volumes of water. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.