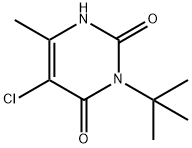
Terbacil
- русский язык имя
- английское имяTerbacil
- CAS №5902-51-2
- CBNumberCB8271017
- ФормулаC9H13ClN2O2
- мольный вес216.66
- EINECS227-595-1
- номер MDLMFCD00055344
- файл Mol5902-51-2.mol
химическое свойство
| Температура плавления | 175-177° |
| плотность | 1.3400 |
| показатель преломления | 1.5388 (estimate) |
| температура хранения | 0-6°C |
| форма | Solid |
| пка | 8.79±0.40(Predicted) |
| цвет | Off-white |
| Растворимость в воде | 0.71g/L(25 ºC) |
| Мерк | 13,9230 |
| БРН | 643054 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | S0017J6E25 |
| Предложение 65 Список | Terbacil |
| Справочник по химии NIST | Terbacil(5902-51-2) |
| Система регистрации веществ EPA | Terbacil (5902-51-2) |
| Коды опасности | Xn |
| Заявления о рисках | 22 |
| РИДАДР | None assigned |
| WGK Германия | 2 |
| RTECS | YQ9360000 |
| Банк данных об опасных веществах | 5902-51-2(Hazardous Substances Data) |
| Токсичность | Approx. LD orally in rats: >5000 mg/kg (Hill) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312+P330:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии. Прополоскать рот.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Terbacil химические свойства, назначение, производство
Химические свойства
Colorless crystals. Mp 175C. Soluble in dimethylacetamide and cyclohexanone; partially soluble in xylene and butyl acetate.Использование
Terbacil is also absorbed by plant roots and inhibits photosynthesis. It is used for the selective control of many annual and some perennial weeds in apples, citrus, peaches, and sugarcane. Application rates range from 0.5 to 8 kg/ha.Общее описание
Colorless crystals. Non corrosive. Used as an herbicide.Реакции воздуха и воды
Water soluble.Профиль реактивности
A uracil derivative.Сельскохозяйственное использование
Herbicide: Terbacil is a selective herbicide that treats a broad spectrum of broadleaf weeds and grasses. It is formulated as a wettable powder and is applied by aircraft or ground equipment on terrestrial food and feed crops (e.g., apples, mint/peppermint/spearmint, sugarcane, and ornamentals), forestry [e.g., cottonwood (forest/shelterbelt)], terrestrialfood (e.g., asparagus, blackberry, boysenberry, dewberry, loganberry, peach, raspberry, youngberry and strawberry), and terrestrial feed (e.g., alfalfa, sainfoin (hay and fodder), and forage). Not approved for use in EU countries. Registered for use in the U.S.Торговое название
COMPOUND® 732; DuPontTM® 732; EXPERIMENTAL HERBICIDE 732; GEONTER®; SINBAR®; TURBSVIL®; ZOBAR®[C]Экологическая судьба
Soil. In microbially active soils, tebuthiuron is degraded via demethylation. The average half-life in soil 12–15 months in areas receiving 60 inches of rainfall a year (Humburg et al., 1989).Groundwater. According to the U.S. EPA (1986) terbacil has a high potential to leach to groundwater.
Plant. The major degradation products of [2-14C]terbacil identified in alfalfa using a mass spectrometer were (% of applied amount): 3-tert-butyl-5-chloro-6-hydroxymethyluracil (11.9) and 6-chloro-2,3-dihydro-7-(hydroxymethyl)-3,3-dimethyl-5H-oxa
Photolytic. Acher et al. (1981) studied the dye-sensitized photolysis of terbacil in aerated aqueous solutions over a wide pH range. After a 2-hour exposure to sunlight, terbacil in aqueous solution (pH range 3.0–9.2) in the presence of methylene blue (3 ppm) or riboflavin (10 ppm), decomposed to 3-tert-5-butyl-5-acetyl-5-hydroxyhydantoin. Deacylation was observed under alkaline conditions (pH 8.0 or 9.2) affording 3-tert-5- hydroxyhydantoin. In neutral or acidic conditions (pH 6.8 or 3.0) containing riboflavin, a mono-N-dealkylated terbacil dimer and an unidentified water-soluble product formed. Product formation, the relative amounts of products formed and the rate of photolysis were found to be all dependent upon pH, sensitizer, temperature and time (Acher et al., 1981).
Chemical/Physical. Stable in water (Worthing and Hance, 1991).
Метаболический путь
When rats are administered terbacil orally, the primary biotransformation products of terbacil in the urine are derived from hydroxylation of the 6-methyl group and are identified as the aglycone, glucuronide, and sulfate. The other conjugated metabolite is the mercapturic acid conjugate of the 6-methyl substituent. Minor metabolites are identified as sulfoxides and sulfone at the 6-methyl substituent of terbacil.Terbacil поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86-755-89396905 +86-15013857715 |
China | 10453 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| 02768782018 18771942761 |
CHINA | 979 | 58 | |
| +86 +8618733132031 |
CHINA | 1303 | 58 | |
| +86-85511178; +86-85511178; |
China | 35425 | 58 |