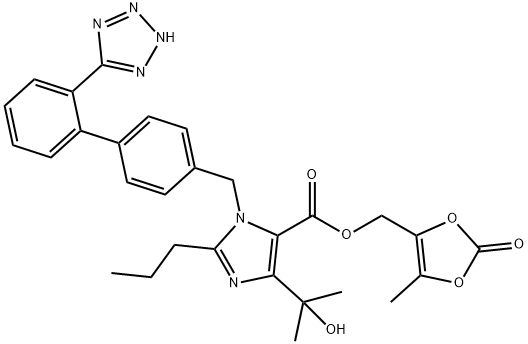
Олмесартана медоксомил
- английское имяOlmesartan Medoxomil
- CAS №144689-63-4
- CBNumberCB8254825
- ФормулаC29H30N6O6
- мольный вес558.59
- EINECS604-433-1
- номер MDLMFCD00944911
- файл Mol144689-63-4.mol
| Температура плавления | 180°C |
| Температура кипения | 804.2±75.0 °C(Predicted) |
| плотность | 1.38±0.1 g/cm3(Predicted) |
| Fp | 180°C |
| температура хранения | 2-8°C |
| растворимость | DMSO: soluble20mg/mL, clear |
| пка | 4.15±0.10(Predicted) |
| форма | powder |
| цвет | white to beige |
| Разложение | 180 ºC |
| ИнЧИКей | UQGKUQLKSCSZGY-UHFFFAOYSA-N |
| SMILES | C1(CCC)N(CC2=CC=C(C3=CC=CC=C3C3=NNN=N3)C=C2)C(C(OCC2=C(C)OC(=O)O2)=O)=C(C(O)(C)C)N=1 |
| Справочник по базе данных CAS | 144689-63-4(CAS DataBase Reference) |
| FDA UNII | 6M97XTV3HD |
| Код УВД | C09CA08 |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302+H312+H332:Вредно при проглатывании, при попадании на кожу или при вдыхании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
Олмесартана медоксомил химические свойства, назначение, производство
Описание
Olmesartan medoxomil was launched in the US as benicar(R), an orally administered treatment for hypertension. Olmesartan, is a new selective and competitive nonpeptide angiotensin II type 1 receptor antagonist and potently inhibits the Ang.ll-induced pressor responses. The drug competitively inhibited binding of [125I1]-All to AT1 receptors in bovine adrenal cortical membranes, but had no effect on binding to AT2 receptors in bovine cerebellar membranes. In comparative clinical studies in patients with essential hypertension, olmesartan reduced sitting cuff diastolic blood pressure significantly more than losartan, valdesartan and ibesartan, while reductions in systolic blood pressure were similar for all treatments. Olmesartan medoxomil was also shown to reduce blood pressure significantly more effectively than losartan and the ACE inhibitor captopril and as effectively as the pbloker atenolol.Химические свойства
White to off-white crystalline powderИспользование
Olmesartan medoxomil is an angiotensin II receptor antagonist used to treat high blood pressure. Olmesartan works by blocking the binding of angiotensin II to the AT1 receptors in vascular muscle. By blocking the binding rather than the synthesis of angiotensin II, olmesartan inhibits the negative regulatory feedback on renin secretion.Olmesartan medoxomil is a pro-drug that is de-esterified to the active metabolite, olmesartan. Olmesartan has a dual method of elimination, with about 60% eliminated by the liver and the remainder by the kidney. In situations of impaired renal or hepatic function, the alternative excretion pathway can compensate for the compromised one. Olmesartan is not metabolized by the cytochrome P450 enzyme system and therefore has a low potential for metabolic drug interactions, a feature that may be of importance when treating patients on multiple drug regimens, such as the elderly. Olmesartan is well tolerated and has an excellent safety profile that is comparable to that of placebo. In addition, olmesartan provides 24-h blood pressure control with a once-daily dosing. In head-to-head studies, olmesartan delivered superior blood pressure reduction when compared with other angiotensin-II receptor antagonists at their recommended doses.
Общее описание
Olmesartan Medoxomil is a synthetic imidazole derivative prodrug with an antihypertensive property. Upon hydrolysis, olmesartan medoxomil is converted to olmesartan. Olmesartan selectively binds to the angiotensin type 1 (AT1) receptor of angiotensin II in vascular smooth muscle and adrenal gland, thereby competing angiotensin II binding to the receptor. This prevents angiotensin II-induced vasoconstriction and decreases aldosterone production, thereby preventing aldosterone-stimulated sodium retention and potassium excretion.Побочные эффекты
Dizziness or lightheadedness may occur as your body adjusts to the medication. If any of these effects persist or worsen, tell your doctor or pharmacist promptly. To reduce the risk of dizziness and lightheadedness, get up slowly when rising from a sitting or lying position.Синтез
Olmesartan Medoxomil can be synthesized in 8 steps from diaminomaleonitrile by successive reactions with trialkylorthopropanoate to access 2-propyl-imidazole-45dicarbonitrile, conversion of the two nitrile functions to the corresponding ethyl esters, followed by methylmagnesium bromide addition to give the corresponding 4-(1-hydroxyalkyl)imidazole derivative.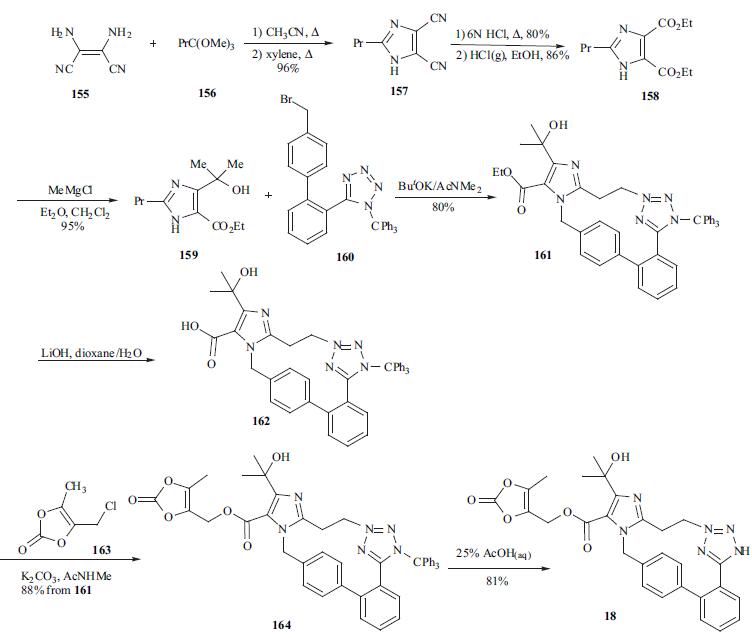
The imidazole ring of olmesartan (18) was constructed with diaminomaleonitrile 155 and trimethylorthobutyrate (156) in CH3CN then xylene to give 157 in 96% yield. Acid hydrolysis of 157 in 6N HCl gave the dicarboxylic acid intermediate. After esterification of the diacid in ethanol in the presence of HCl, diester 158 was treated with MeMgCl to give 4-(1-hydroxyalkyl) imidazole 159 in 95% yield. Alkylation of 159 with biphenyl bromide 160 in the presence of potassium tbutoxide afforded 161 in 80% yield. Ester 161 was then hydrolyzed to free carboxylic acid 162 under basic conditions, and 162 was treated with chloride 163 in the presence of K2CO3 to give ester 164 in 88% yield from 161.Lastly, the trityl group was removed with 25% aqueous acetic acid to give olmesartan (18) in 81% yield.
Олмесартана медоксомил запасные части и сырье
Олмесартана медоксомил поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8615065888978 | China | 92 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| 0592-5800732; +8613806035118 |
China | 988 | 58 | ||
| +86-13176845580 +86-13176845580 |
China | 232 | 58 | ||
| +8619956560829 | China | 286 | 58 | ||
| +8615689548120 | China | 193 | 58 | ||
| +8617756083858 | China | 973 | 58 | ||
| +86-0086-57187702781 +8613675893055 |
China | 295 | 58 | ||
| +8615350851019 | China | 1001 | 58 | ||
| 010-60279497 | CHINA | 1803 | 55 |