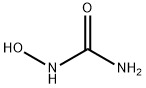
Гидроксикарбамид
- английское имяHydroxyurea
- CAS №127-07-1
- CBNumberCB8249322
- ФормулаCH4N2O2
- мольный вес76.05
- EINECS204-821-7
- номер MDLMFCD00007943
- файл Mol127-07-1.mol
химическое свойство
| Температура плавления | 135-140 °C |
| Температура кипения | 136.04°C (rough estimate) |
| плотность | 1.457±0.06 g/cm3(Predicted) |
| показатель преломления | 1.4840 (estimate) |
| температура хранения | 2-8°C |
| растворимость | H2O: 50 mg/mL |
| форма | powder |
| пка | 10.56±0.23(Predicted) |
| цвет | white |
| Запах | odorless or almost odorless |
| Растворимость в воде | soluble |
| Мерк | 14,4848 |
| БРН | 1741548 |
| Стабильность | Stable for 2 years as supplied from date of purchase. Solutions in water may be stored at -20°C for up to 3 months. |
| Справочник по базе данных CAS | 127-07-1(CAS DataBase Reference) |
| Словарь онкологических терминов NCI | Droxia; Hydrea; hydroxyurea |
| FDA UNII | X6Q56QN5QC |
| Словарь наркотиков NCI | Droxia |
| Код УВД | L01XX05 |
| Предложение 65 Список | Hydroxyurea |
| МАИР | 3 (Vol. 76) 2000 |
| Система регистрации веществ EPA | Hydroxyurea (127-07-1) |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
больше
| Коды опасности | T,Xn | |||||||||
| Заявления о рисках | 46-63-61-40 | |||||||||
| Заявления о безопасности | 53-36/37-45-36-22 | |||||||||
| РИДАДР | 2811 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | YT4900000 | |||||||||
| Примечание об опасности | Toxic | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29242100 | |||||||||
| Банк данных об опасных веществах | 127-07-1(Hazardous Substances Data) | |||||||||
| Токсичность | dog,LD50,intravenous,> 1gm/kg (1000mg/kg),Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H361:Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению или на неродившегося ребенка.
H340:Может вызывать генетические дефекты.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Гидроксикарбамид химические свойства, назначение, производство
Химические свойства
Off-White Crystalline SolidИспользование
An anti-neoplastic - inhibits ribonucleoside reductase and DNA replication. A potential therapy for sickle cell anemia which involves the nitrosylation of sickle cell hemoglobin. Horseradish peroxidase catalyzes nitric oxide formation from hydroxyurea in the presence of hydrogen peroxide.Показания
Hydroxyurea (Hydrea) inhibits the enzyme ribonucleotide reductase and thus depletes intracellular pools of deoxyribonucleotides, resulting in a specific impairment of DNA synthesis. The drug therefore is an Sphase specific agent whose action results in an accumulation of cells in the late G1- and early S-phases of the cell cycle.Общее описание
HONH-CO-NH2. The drug is available in a 500-mg capsulefor oral use. Hydroxyurea is often considered an antimetabolitedrug, and it is used to treat myelogenousleukemia, ovarian cancer, and essential thrombocytosis. Themechanism of action of hydroxyurea involves inhibition ofDNA biosynthesis by inhibition of the enzyme ribonucleotidereductase). Resistance can occur viaincreased expression of ribonucleotide reductase. The oralbioavailability is quite high approaching 100% and the drugis distributed to all tissues. Hydroxyurea readily enters theCNS and distributes to human breast milk. A major portionof the total dose is excreted unchanged in the urine. Thedrug has been shown to increase the toxicity of 5-FU, andhydroxyurea may increase the effectiveness of some antimetaboliteHIV drugs. The toxicity profile includes myelosuppression,leucopenia, nausea, vomiting, pruritus hyperpigmentation,headache, drowsiness, and confusion.Реакции воздуха и воды
Water soluble.Профиль реактивности
An amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).Пожароопасность
Flash point data for Hydroxyurea are not available; however, Hydroxyurea is probably combustible.Механизм действия
Hydroxyurea is rapidly absorbed after oral administration, with peak plasma levels achieved approximately 1 to 2 hours after drug administration; its elimination half-life is 2 to 3 hours. The primary route of excretion is renal, with 30 to 40% of a dose excreted unchanged.Клиническое использование
Hydroxyurea is used for the rapid lowering of blood granulocyte counts in patients with chronic granulocytic leukemia. The drug also can be used as maintenance therapy for patients with the disease who have become resistant to busulfan. Only a small percentage of patients with other malignancies have had even brief remissions induced by hydroxyurea administration.Побочные эффекты
Hematological toxicity, with white blood cells affected more than platelets, may occur. Megaloblastosis of the bone marrow also may be observed. Recovery is rapid, generally within 10 to 14 days after discontinuation of the drug. Some skin reactions, including hyperpigmentation and hyperkeratosis, have been reported with chronic treatment.Метаболизм
Hydroxyurea has excellent oral bioavailability (80–100%), and serum levels peak within 2 hours of consuming the capsules. If a positive response is noted within 6 weeks, toxicities generally are mild enough to permit long-term or indefinite therapy on either a daily or every-3-day basis. Leukopenia and, less commonly, thrombocytopenia and/or anemia are the most serious adverse effects. Excretion of the unchanged drug and the urea metabolite is via the kidneys. The carbon dioxide produced as a by-product of hydroxyurea metabolism is excreted in the expired air.Методы очистки
Recrystallise hydroxyurea from absolute EtOH (10g in 150mL). Note that the rate of solution in boiling EtOH is slow (15-30minutes). It should be stored in a cool dry place, but some decomposition could occur after several weeks. [Deghenghi Org Synth Coll Vol V 645 1973.] It is very soluble in H2O and can be crystallised from Et2O. [Kfod Acta Chem Scand 10 256 1956, Beilstein 3 IV 170.]Гидроксикарбамид запасные части и сырье
Гидроксикарбамид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-15531157085 +86-15531157085 |
China | 8808 | 58 | |
| +86-13131129325 | China | 5872 | 58 | |
| +86-18600796368 +86-18600796368 |
China | 485 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29730 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21630 | 55 | |
| +8615102730682 | CHINA | 566 | 55 | |
| 025-85710122 17714198479 | CHINA | 885 | 55 | |
| +undefined-21-51877795 | China | 33024 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29862 | 58 | |
| +86 18953170293 | China | 2930 | 58 |