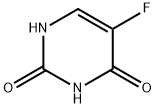
5-фторурацил
- английское имя5-Fluorouracil
- CAS №51-21-8
- CBNumberCB8162744
- ФормулаC4H3FN2O2
- мольный вес130.08
- EINECS200-085-6
- номер MDLMFCD00006018
- файл Mol51-21-8.mol
| Температура плавления | 282-286 °C (dec.) (lit.) |
| Температура кипения | 190-200°C/0.1mmHg |
| плотность | 1.4593 (estimate) |
| температура хранения | 2-8°C |
| растворимость | H2O: 10 mg/mL, clear |
| форма | powder |
| пка | pKa 8.0±0.1 (H2O) (Uncertain);3.0±0.1(H2O) (Uncertain) |
| цвет | white |
| РН | 4.3-5.3 (10g/l, H2O, 20℃) |
| Растворимость в воде | 12.2 g/L 20 ºC |
| Чувствительный | Air Sensitive |
| Мерк | 14,4181 |
| БРН | 127172 |
| Стабильность | Stable. Light sensitive. Combustible. Incompatible with strong oxidizing agents, strong bases. |
| ИнЧИКей | GHASVSINZRGABV-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 51-21-8(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| Словарь онкологических терминов NCI | 5-fluorouracil; 5-FU; Efudex; Fluoroplex; fluorouracil |
| FDA UNII | U3P01618RT |
| Словарь наркотиков NCI | Efudex |
| Код УВД | L01BC02,L01BC52 |
| Предложение 65 Список | Fluorouracil |
| МАИР | 3 (Vol. 26, Sup 7) 1987 |
| Справочник по химии NIST | 2,4-Pyrimidinedione, 5-fluoro-(51-21-8) |
| Система регистрации веществ EPA | 5-Fluorouracil (51-21-8) |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
| Коды опасности | Xn,T,C,Xi | |||||||||
| Заявления о рисках | 22-20/21/22-52-25 | |||||||||
| Заявления о безопасности | 36-36/37-36/37/39-22-45-26 | |||||||||
| РИДАДР | UN 2811 6.1/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | YR0350000 | |||||||||
| F | 10-23 | |||||||||
| Примечание об опасности | Irritant/Highly Toxic | |||||||||
| TSCA | T | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29335995 | |||||||||
| Банк данных об опасных веществах | 51-21-8(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 230 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
5-фторурацил химические свойства, назначение, производство
Описание
5-Fluorouracil (5-FU) is a prodrug form of the thymidylate synthase inhibitor fluorodeoxyuridylate (FdUMP). It is also converted to the active metabolites FUTP and FdUTP, which induce RNA and DNA damage, respectively. In vivo, 5-FU (15 mg/kg) when administered in combination with docetaxel reduces tumor growth in B88 and CAL 27 oral squamous cell carcinoma (OSCC) mouse xenograft models. Formulations containing 5-FU have been used in the treatment of colorectal, breast, gastric, and pancreatic cancers.Химические свойства
White or almost white, crystalline powderИспользование
5-Fluorouracil is used as an antitumor agent in the treatment of anal, breast, colorectal, oesophageal, stomach, pancreatic and skin cancers. It finds application as a suicide inhibitor due to its irreversible inhibition of thymidylate synthase. It is also used in the treatment of actinic keratoses and bowen's disease. Further, it serves as a potent antineoplastic agent in clinical use. In addition to this, it acts as a DNA synthesis inhibitor.Показания
Fluorouracil (5-fluorouracil, 5-fluorouracil, Efudex, Adrucil) is a halogenated pyrimidine analogue that must be activated metabolically. The active metabolite that inhibits DNA synthesis is the deoxyribonucleotide 5-fluoro-2'deoxyuridine-S'-phosphate (FdUMP). 5- Fluorouracil is selectively toxic to proliferating rather than non-proliferating cells and is active in both the G1- and S-phases. The target enzyme inhibited by 5-fluorouracilfluorouracil is thymidylate synthetase.methylenetetrahydrofolate dihydrofolate The carbon-donating cofactor for this reaction is N5,N10 methylenetetrahydrofolate, which is converted to dihydrofolate. The reduced folate cofactor occupies an allosteric site on thymidylate synthetase, which allows for the covalent binding of 5-FdUMP to the active site of the enzyme.
Общее описание
White to nearly white crystalline powder; practically odorless. Used as an anti neoplastic drug, chemosterilant for insects.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
5-Fluorouracil may be sensitive to prolonged exposure to light. Solutions discolor on storage. 5-Fluorouracil can react with oxidizing agents and strong bases. Incompatible with methotrexate sodium.Опасность
Questionable carcinogen.Угроза здоровью
Minimum toxic dose in humans is approximately 450 mg/kg (total dose) over 30 days for the ingested drug. Intravenous minimum toxic dose in humans is a total dose of 6 mg/kg over three days. Depression of white blood cells occurred after intravenous administrative of a total dose of 480 mg/kg over 32 days. Occasional neuropathy and cardiac toxicity have been reported. Do not use during pregnancy. Patients with impaired hepatic or renal function, with a history of high-dose pelvic irradiation or previous use of alkylating agents should be treated with extreme caution. Patients with nutritional deficiencies and protein depletion have a reduced tolerance to 5-Fluorouracil.Пожароопасность
Emits very toxic fumes of flourides and nitrogen oxides when heated to decomposition. Avoid decomposing heat.Биологическая активность
Anticancer agent. Metabolized to form fluorodeoxyuridine monophosphate (FdUMP), fluorodeoxyuridine triphosphate (FdUTP) and fluorouridine (FUTP). FdUMP inhibits thymidylate reductase causing a reduction in dTMP synthesis. FUTP and FdUTP are misincorporated into RNA and DNA respectively.Механизм действия
Another action proposed for 5-fluorouracil may involve the incorporation of the nucleotide 5-fluorouridine triphosphate (5-FUTP) into RNA. The cytotoxic role of these “fraudulent” 5-fluorouracil-containing RNAs is not well understood.Several possible mechanisms of resistance to 5-fluorouracil have been identified, including increased synthesis of the target enzyme, altered affinity of thymidylate synthetase for FdUMP, depletion of enzymes (especially uridine kinase) that activate 5-fluorouracil to nucleotides, an increase in the pool of the normal metabolite deoxyuridylic acid (dUMP), and an increase in the rate of catabolism of 5-fluorouracil.
The drug has been administered orally, but absorption by this route is erratic. The plasma half-life of 5- fluorouracil after intravenous injection is 10 to 20 minutes. It readily enters CSF. Less than 20% of the parent compound is excreted into the urine, the rest being largely metabolized in the liver.
Фармаколо?гия
Local inflammatory reactions characterized by erythema, edema, crusting, burning, and pain are common (and, some would argue, desirable) but may be minimized by reduced frequency of application or use in combination with a topical corticosteroid.Клиническое использование
5-Fluorouracil (FU) is widely used in the treatment of a range of cancers including breast and cancers of the aerodigestive tract, but has had the greatest impact in colorectal cancer. 5-FU-based chemotherapy improves overall and disease-free survival of patients with resected stage III colorectal cancer. Nonetheless, response rates for 5-FU-based chemotherapy as a first-line treatment for advanced colorectal cancer are only between 10 and 15%. Combination of 5-FU with newer chemotherapies, such as irinotecan and oxaliplatin, has improved the response rates for advanced colorectal cancer to between 40 and 50%.Побочные эффекты
Patients who are genetically deficient in this enzyme will experience a more pronounced effect from this drug and are at significant risk for use-limiting toxicity. In general, women clear fluorouracil faster than men do. Dosage adjustments usually are not required in hepatic or renal dysfunction. Major toxicities are related to bone marrow depression, stomatitis/esophagopharyngitis, and potential GI ulceration. Nausea and vomiting are common. Solutions of fluorouracil are light sensitive, but discolored products that have been properly stored and protected from light are still safe to use.Профиль безопасности
Poison by ingestion, intraperitoneal, subcutaneous, and intravenous routes. Moderately toxic by parented and rectal routes. Experimental teratogenic and reproductive effects. Human systemic effects: EKG changes, bone marrow changes, cardiac, pulmonary, and gastrointestinal effects. Human mutation data reported. A human skin irritant. Questionable carcinogen. When heated to decomposition it emits very toxic fumes of Fand NOx.Возможный контакт
This material is used as an antineo plastic drug for cancer treatment and as a chemosterilant for insects.Перевозки
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, methotrexrate sodium, sources of heat.5-фторурацил запасные части и сырье
сырьё
1of2
запасной предмет
1of2
5-фторурацил поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0552-4929304 +86-18055277008 |
China | 57 | 55 | ||
| +86-010-60605551 +86-18046518538 |
China | 321 | 58 | ||
| +8613651027935 | China | 191 | 58 | ||
| +8613343730176 | China | 83 | 58 | ||
| +8617774091612 | China | 129 | 58 | ||
| +86-0086-0373-3088722 +8618637352520 |
China | 94 | 58 | ||
| +86-020-61855200-902 +8618124244216 |
China | 897 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 |