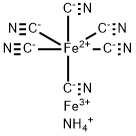
Аммоний железо (III) гексацианоферрат (II) гидра
- английское имяAMMONIUM IRON (III) HEXACYANOFERRATE (II)
- CAS №25869-00-5
- CBNumberCB7310892
- ФормулаC6H4Fe2N7
- мольный вес285.84
- EINECS247-304-1
- номер MDLMFCD01726733
- файл Mol25869-00-5.mol
химическое свойство
| RTECS | LJ8140000 |
| Растворимость в воде | Soluble in water and mineral acids. Insoluble in alkali. |
| Мерк | 14,7910 |
| Пределы воздействия | ACGIH: TWA 1 mg/m3 NIOSH: IDLH 25 mg/m3; TWA 1 mg/m3 |
| LogP | -0.250 (est) |
| FDA 21 CFR | 73.1298; 73.2298 |
| FDA UNII | 9R0NVI936I |
| Система регистрации веществ EPA | Ferric ammonium ferrocyanide (25869-00-5) |
| Заявления о рисках | 20/21/22 | |||||||||
| Заявления о безопасности | 22-36/37/39 | |||||||||
| TSCA | Yes | |||||||||
| Токсичность | LD50 orally in mice: >5 g/kg (Pearce) | |||||||||
| NFPA 704: |
|
Аммоний железо (III) гексацианоферрат (II) гидра химические свойства, назначение, производство
Использование
It is employed as a intermediate for pharmaceuticals. Also used in decorative cosmetics.Общее описание
Solid.Реакции воздуха и воды
Irradiation of aqueous solutions of the compound will liberate hydrocyanic acid.Профиль реактивности
AMMONIUM IRON (III) HEXACYANOFERRATE (II) is an inorganic cyanide. Inorganic cyanides react slowly with water to evolve gaseous hydrogen cyanide (HCN). Acids cause the rapid evolution of HCN; carbon dioxide from the air is sufficiently acidic to liberate HCN from solutions of cyanides. Inorganic cyanides are incompatible with isocyanates, nitrides, and peroxides. Cyanides have been known to initiate polymerization reactions of epoxides. Cyanides form compounds with metal salts; heat and hydrogen production may accompany these reactions. Some cyanides can detonate when exposed to shock, heat, or friction. In the presence of strong acids or acid fumes may release highly toxic fumes of cyanides.Пожароопасность
Flash point data for AMMONIUM IRON (III) HEXACYANOFERRATE (II) are not available. AMMONIUM IRON (III) HEXACYANOFERRATE (II) is probably combustible.Аммоний железо (III) гексацианоферрат (II) гидра поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| +8618058761490 | China | 49983 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| 021-61259108 18621169109 |
China | 40228 | 62 | |
| 400-6106006 | China | 30123 | 84 | |
| 021-021-58432009 400-005-6266 |
China | 44689 | 61 | |
| 021-60345187 13671753212 |
China | 10274 | 55 | |
| 86 512-62572107 62962707 | China | 800 | 58 |