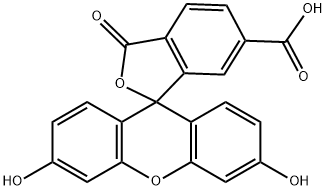
6-карбоксифлуоресцеин
- английское имя6-Carboxyfluorescein
- CAS №3301-79-9
- CBNumberCB7288380
- ФормулаC21H12O7
- мольный вес376.32
- EINECS636-503-2
- номер MDLMFCD00036873
- файл Mol3301-79-9.mol
химическое свойство
| Температура плавления | >300 °C(lit.) |
| Температура кипения | 736.4±60.0 °C(Predicted) |
| плотность | 1.73±0.1 g/cm3(Predicted) |
| температура хранения | Sealed in dry,2-8°C |
| растворимость | DMSO: soluble |
| форма | Solid |
| пка | 3.3, 4.6, 6.4, 7.0(at 25℃) |
| цвет | Yellow to orange |
| Водородный показатель | Weak green ' uorescence (6.0) to strong green ' uourescence (7.2) |
| λмакс | 492nm |
| БРН | 54341 |
| Основное приложение | Diagnosis of hematologic cancer, nongastric diseases, detection of genetically modified wheat, chromosomes, gene expression, nucleic acid, hepatitis A virus, avian influenza virus subtype H5 and H5N1, SARS virus, herpex simplex virus |
| Биологические приложения | Diagnosing bladder cancer; evaluating corneal endothelial barrier function; as a substrate for measuring protein kinase activity, methyltransferase activity, phospholipase activity, rhodanese activity,sulfotransferase activity;use in ophthalmology |
| Справочник по базе данных CAS | 3301-79-9(CAS DataBase Reference) |
| FDA UNII | JHJ853P6SM |
| Система регистрации веществ EPA | Spiro[isobenzofuran-1(3H),9'-[9H]xanthene]-6-carboxylic acid, 3',6'-dihydroxy-3-oxo- (3301-79-9) |
| UNSPSC Code | 12352005 |
| NACRES | NA.47 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 26-36/37 | |||||||||
| WGK Германия | 3 | |||||||||
| F | 10-21 | |||||||||
| кода HS | 3204 90 00 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
6-карбоксифлуоресцеин химические свойства, назначение, производство
Химические свойства
Orange SolidИспользование
6-Carboxyfluorescein (6-FAM) is derivative of fluorescein giving stable derivative upon conjugation with biopolymers.It has been used to check the plasma membrane integrity in sperm cells. The single isomer, 6-FAM, contains a carboxylic acid that can be used to react with primary amines via carbodiimide activation of the carboxylic acid. Commercially available FAM is a mixture of two isomers, 5-FAM and 6-FAM, and the correct name is 5(6)-carboxyfluorescein.Подготовка
1,2,4-Benzenetricarboxylic anhydride (also called 4- carboxyphthalic anhydride, 25.0 g, 0.13 mol) was added to a solution of 1,3-dihydroxybenzene (also called resorcinol, 28.6 g, 0.26 mol) in methane sulfonic acid (1M). An air condenser was attached to the flask and the reaction was heated at 85 oC in an open vessel for 24 h. After cooling to room temperature, the reaction mixture was poured into 7 volumes of ice/water. An orange-yellow precipitate formed; this was collected by filtration and dried in an oven at 200 oC. This residue was recrystallized two times from methanol/hexane to give 1.0 g of 6- carboxyfluorescein methanesulfonic acid adduct preferentially crystallizes from MeOH/hexanes. The mother liquors from this procedure were collected, the solvent was removed in vacuo, and the residues were recrystallized two times from ethanol/hexanes to give 3.2 g of 5-carboxyfluorescein methanesulfonic acid adduct preferentially crystallizes from EtOH/hexanes. Finally, the mother liquors from this experiment were combined, evaporated to dryness, and recrystallized two times from methanol/hexanes to give another 3.0 g of 6-carboxyfluorescein methanesulfonic acid adduct preferentially crystallizes from MeOH/hexanes, making a total yield of 4.0 g, 40 %. Careful dropwise addition of conc. HCl(aq) to solutions of these methanesulfonic esters preferentially crystallizes from EtOH/hexanes and preferentially crystallizes from MeOH/hexanes in 4M sodium hydroxide gave 5-carboxyfluorescein and 6-carboxyfluorescein, respectively in near quantitative yield.Определение
ChEBI: 6-carboxyfluorescein is a monocarboxylic acid. It derives from a fluorescein (lactone form).прикладной
6-Carboxyfluorescein is a useful reagent for the preparation of hydrolytically stable fluorescent conjugates and is a useful starting material for the synthesis of other fluorescein-derives reagent. Fluorescein is the most common fluorescent derivatization reagent for labeling biomolecules. In addition to its relatively high absorptivity, excellent fluorescence quantum yield, and good water solubility, fluorescein has an excitation maximum that closely matches the 488 nm spectral line of the argon-ion laser.Биохимия/физиол Действия
6-Carboxyfluorescein diacetate is a membrane permeable compound. It can be hydrolysed by intracellular esterases to form a membrane impermeable bright green fluorescent molecule, 6-carboxyfluorescein.6-карбоксифлуоресцеин запасные части и сырье
сырьё
запасной предмет
6-карбоксифлуоресцеин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +undefined18915499068 | China | 120 | 58 | ||
| +86-029-81138252 +86-18789408387 |
China | 3889 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +8613343047651 | China | 3692 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 025-83697070 | CHINA | 3009 | 60 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| +86 519 86305871 | CHINA | 4241 | 58 | ||
| +1-858-6993322 | United States | 18526 | 58 |