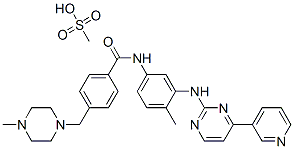
Imatinib mesylate
- русский язык имя
- английское имяImatinib mesylate
- CAS №220127-57-1
- CBNumberCB7173646
- ФормулаC30H35N7O4S
- мольный вес589.71
- EINECS606-892-3
- номер MDLMFCD04307699
- файл Mol220127-57-1.mol
химическое свойство
| Температура плавления | 214-224°C |
| температура хранения | 2-8°C |
| растворимость | H2O: soluble10mg/mL, clear |
| форма | White solid |
| цвет | white to beige |
| Растворимость в воде | water: 100mg/mL |
| Стабильность | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or water may be stored at -20°C for up to 3 months. |
| ИнЧИКей | YLMAHDNUQAMNNX-UHFFFAOYSA-N |
| SMILES | C(NC1=CC=C(C)C(NC2=NC=CC(C3=CC=CN=C3)=N2)=C1)(=O)C1=CC=C(CN2CCN(C)CC2)C=C1.CS(O)(=O)=O |
| Справочник по базе данных CAS | 220127-57-1(CAS DataBase Reference) |
| FDA UNII | 8A1O1M485B |
| Словарь онкологических терминов NCI | Gleevec; imatinib mesylate; STI571 |
| Словарь наркотиков NCI | Gleevec |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | CV5520550 | |||||||||
| кода HS | 29350090 | |||||||||
| Банк данных об опасных веществах | 220127-57-1(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H361d:Предполагается, что данное вещество может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Imatinib mesylate химические свойства, назначение, производство
Описание
In May 2001, the FDA approved imatinib as a new cancer drug after a record review time of just 2.5 months. Imatinib was launched as Gleevec in the US for chronic myelogenous leukemia (CML) in blast crisis, accelerated phase or chronic phase after interferon-alpha failure. This compound can be prepared by a four step sequence from a condensation of the 1-(3-pyridyl)ethanone with dimethyl formamide dimethylacetal, followed by successive cyclization with the methyl-nitrophenyl guanidine, hydrogenolysis and condensation with the benzoyl chloride of the methylpiperazine. Imatinib is the first of a new class of anticancer drugs that are specifically designed to target the molecular pathways involved (oncogenic event) in the development of disease. The Brc-Abl oncoprotein is a constitutively active tyrosine kinase that causes CML. Imatinib is a competitive inhibitor of this tyrosine kinase as well as Abl, Kit and the PDGFR kinases It binds to the ATP-binding site of the target kinase and prevents the transfer of phosphate from ATP to the tyrosine residues of various substrates and consequently blocks the proliferation of the leukemic cells. Phase II studies demonstrated that in chronic phase CML, over 90% of the patients had their leukocyte counts return to normal and 56% had a major cytogenic response. No phase III data is currently available. It is clear from the evidence available that imatinib has advantages over IFN-alpha, such as reduced toxicity, more rapid hematological response, higher rate of cytogenic response and oral administration. The drug is well tolerated, producing few side effects, classified as grade 1 nausea, muscle cramps, diarrhea, edema and vomiting. Imatinib is metabolized primarily by the CYP3A4 enzyme system and drugs capable of modulating this system would be expected to modify the patient's exposure. Novartis expects to launch imatinib for the treatment of gastrointestinal stromal tumors in 2002.Химические свойства
Off-White SolidИспользование
Imatinib Mesylate is orally bioavailability mesylate salt of Imatinib, which is a multi-target inhibitor of v-Abl, c-Kit and PDGFR with IC50 of 0.6 μM, 0.1 μM and 0.1 μM, respectively. Imatinib also known as Gleevec, Glivec, CGP-57148B, STI-571 & ImatinibОпределение
ChEBI: A methanesulfonate (mesylate) salt that is the monomesylate salt of imatinib. Used for treatment of chronic myelogenous leukemia and gastrointestinal stromal tumours.Общее описание
Imatinib is available in 100- and 400-mg capsules for oraladministration and is indicated for the treatment of CML,gastrointestinal stromal tumors (GIST) that express Kit andacute lymphoplastic leukemia that is positive for thePhiladelphia chromosome.Bioavailability of the agent is nearly 100% by the oralroute. The agent is highly protein bound and metabolized tothe N-desmethyl derivative by CYP3A4-mediated removalof the piperazinyl methyl group. The resulting metabolite issimilar to the parent in activity. Elimination occurs primarilyin the feces, and the terminal half-life is 18 hours forthe parent and 40 hours of the N-desmethyl metabolite.Resistant forms of the TK are known, which have alteredamino acids that prevent binding. In addition, there may beincreased levels of the kinase itself. The drug is also a substratefor Pgp and an additional efflux transporter known asbreast cancer resistance protein (BCRP), both of which removethe drug from the cell. These transporters are also inhibitedby the agent as well. Severe side effects include ascites,neutropenia, thrombocytopenia, skin rash, andpulmonary edema. Less serious side effects include nausea/vomiting, heartburn, and headache but overall, the agentis better tolerated than most other medications used in treatingthe disease.
Imatinib mesylate поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| 0592-5800732; +8613806035118 |
China | 988 | 58 | |
| +86-53169958659 +86-13153181156 |
China | 294 | 58 | |
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | |
| +86-25-84696168 +86-15380713688 |
China | 2428 | 58 | |
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +8618092446649 | China | 1143 | 58 | |
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 |