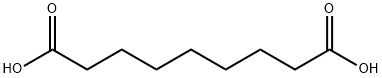
Азелаиновая кислота
- английское имяAzelaic acid
- CAS №123-99-9
- CBNumberCB6852982
- ФормулаC9H16O4
- мольный вес188.22
- EINECS204-669-1
- номер MDLMFCD00004432
- файл Mol123-99-9.mol
| Температура плавления | 98 °C |
| Температура кипения | 286 °C100 mm Hg(lit.) |
| плотность | 1,029 g/cm3 |
| плотность пара | 6.5 (vs air) |
| давление пара | <1 mm Hg ( 20 °C) |
| показатель преломления | 1.4303 |
| Fp | 215 °C |
| температура хранения | Store below +30°C. |
| растворимость | 2.4g/l |
| форма | Slightly Crystalline Powder or Flakes |
| пка | 4.53, 5.33(at 25℃) |
| цвет | White to slightly yellow |
| РН | 3.5 (1g/l, H2O) |
| Растворимость в воде | 2.4 g/L (20 ºC) |
| Мерк | 14,905 |
| БРН | 1101094 |
| Стабильность | Stable. Combustible. Incompatible with bases, strong oxidizing agents. Readily biodegrades in soil and water with >70% DOC reduction after 28 days. |
| ИнЧИКей | BDJRBEYXGGNYIS-UHFFFAOYSA-N |
| LogP | 1.57 at 25℃ |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | AZELAIC ACID |
| FDA 21 CFR | 175.105; 175.300; 175.320 |
| Справочник по базе данных CAS | 123-99-9(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | F2VW3D43YT |
| Код УВД | D10AX03 |
| Справочник по химии NIST | Nonanedioic acid(123-99-9) |
| Система регистрации веществ EPA | Azelaic acid (123-99-9) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 24/25-36-26 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | CM1980000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29171390 | |||||||||
| Банк данных об опасных веществах | 123-99-9(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: > 4000 mg/kg LD50 dermal Rat > 10 g/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P332+P313:При возникновении раздражения кожи: обратиться за медицинской помощью.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
Азелаиновая кислота химические свойства, назначение, производство
Описание
Azelaic acid is a topical antiacne agent which exerts its therapeutic action through a myriad of antimicrobial, antiproliferative and cytostatic effects. In vitro, azelaic acid hasbeen shown to inhibit DNA polymerases in several tumor cell lines.Химические свойства
Azelaic acid is an organic compound with the formula (CH2)7(CO2H)2. This saturated dicarboxylic acid exists as a white powder. It is found in wheat, rye, and barley. It is a component of a number of hair and skin conditioners.Использование
Azelaic acid is used in lacquers, alkyd resins, plasticizers, adhesives, polyamides, urethane elastomers, and organic syntheses. Azelaic acid is also used in treating of acne.Методы производства
Azelaic acid is industrially produced by the ozonolysis of oleic acid. The side product is nonanoic acid. It is produced naturally by Malassezia furfur (also known as Pityrosporum ovale), a yeast that lives on normal skin. The bacterial degradation of nonanoic acid gives azelaic acid.Показания
Azelaic acid (Azelex) is a naturally occurring dicarboxylic acid produced by the yeast Malassezia furfur. Azelaic acid inhibits tyrosinase, a rate-limiting enzyme in the synthesis of the pigment melanin. This may explain why diminution of melanin pigmentation occurs in the skin of some patients with pityriasis versicolor, a disease caused by M. furfur. Azelaic acid is bacteriostatic against a number of species thought to participate in the pathogenesis of acne, including Propionibacterium acnes. The drug may also reduce microcomedo formation by promoting normalization of epidermal keratinocytes.Подготовка
Azelaic acid is made by the ozonolysis of oleic acid: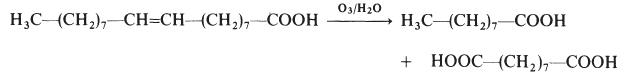
прикладной
Azelaic acid, also known as azalea acid, is a white to slightly yellow powder. Azelaic acid is a medium-long chain dibasic acid[1]. In recent years, with the rapid development of the organic synthetic chemical industry, the demand for medium and long chain dibasic acids is increasing. The medium and long chain dibasic acids and their derivatives have a wide range of industrial applications and a broad product market.Общее описание
Azelaic acid is used as a therapeutic agent in dermatology.Механизм действия
Naturally occurring dicarboxylic acid that is bacteriostatic to Propionibacterium acnes. It also decreases conversion of testosterone to 5{pi}ga-dihydrotestosterone (DHT) and alters keratinization of the microcomedone. It may also be beneficial in the treatment of melasma. The mechanism of action is not fully understood. Deoxyribonucleic acid (DNA) synthesis is reduced, and mitochondrial cellular energy products are inhibited in melanocytes.Приложение биотехнологий?
In plants, azelaic acid serves as a "distress flare" involved in defense responses after infection. It serves as a signal that induces the accumulation of salicylic acid, an important component of a plant's defensive response.Клиническое использование
Azelaic acid is used for the treatment of mild to moderate acne, particularly in cases characterized by marked inflammation-associated hyperpigmentation.Профиль безопасности
Low toxicity by ingestion. A skinand eye irritant. Closely related to glutaric acid and adipicacid. Combustible when exposed to heat or flame; canreact with oxidizing materials.Методы очистки
Recrystallise it from H2O(charcoal) or thiophene-free *benzene. The acid can be dried by azeotropic distillation with toluene, the residual toluene solution is then cooled and filtered, and the precipitate is dried in a vacuum oven. It has been purified by zone refining or by sublimation onto a cold finger at 10-3torr. It distils above 360o with partial formation of the anhydride. The dimethyl ester has m –3.9o and b 140o/8mm. [Hill & McEwen Org Synth Coll Vol II 53 1943, Beilstein 2 IV 2055.]Азелаиновая кислота запасные части и сырье
сырьё
1of2
запасной предмет
1of2
Азелаиновая кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | ||
| +8615309206328 | China | 296 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-15271838296; +8615271838296 |
China | 1512 | 58 | ||
| +8618629063126 | China | 435 | 58 | ||
| 0592-5800732; +8613806035118 |
China | 988 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-86-4001020630 +8619831957301 |
China | 1000 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 |