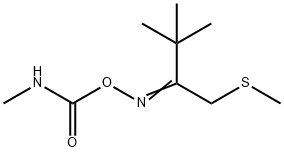
THIOFANOX
- русский язык имя
- английское имяTHIOFANOX
- CAS №39196-18-4
- CBNumberCB6699581
- ФормулаC9H18N2O2S
- мольный вес218.32
- EINECS254-346-4
- номер MDLMFCD00055322
- файл Mol39196-18-4.mol
| Температура плавления | 57℃ |
| плотность | 1.1664 (rough estimate) |
| давление пара | 2.3×10-2 Pa (25 °C) |
| показатель преломления | 1.6800 (estimate) |
| Fp | >100 °C |
| температура хранения | 0-6°C |
| Растворимость в воде | 5.2 g 1 l-1 (22 °C) |
| пка | 13.82±0.46(Predicted) |
| Рейтинг продуктов питания EWG | 1-2 |
| FDA UNII | 9ZE4QF28IN |
| Система регистрации веществ EPA | Thiofanox (39196-18-4) |
| Коды опасности | T+,N |
| Заявления о рисках | 27/28-50/53 |
| Заявления о безопасности | 27-36/37-45-60-61 |
| РИДАДР | 2757 |
| WGK Германия | 3 |
| RTECS | EL8200000 |
| Класс опасности | 6.1(a) |
| Группа упаковки | II |
| кода HS | 29309090 |
| Банк данных об опасных веществах | 39196-18-4(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H300+H310:Смертельно при проглатывании или при контакте с кожей.
-
оператор предупредительных мер
P262:Избегать попадания в глаза, на кожу или одежду.
P264:После работы тщательно вымыть кожу.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P302+P352+P310:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Немедленно обратиться за медицинской помощью.
THIOFANOX химические свойства, назначение, производство
Химические свойства
Thiofanox is a colorless solid with a pungent odor.Использование
Thiofanox is a systemic soil insecticide used for the control of aphids, mites, thrips, plant bugs, leafhoppers and beetles in/on sugar beet and potatoes.Общее описание
THIOFANOX is a colorless solid with a pungent odor. Used as a systemic insecticide and acaricide.Реакции воздуха и воды
Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids.Профиль реактивности
THIOFANOX is a thiocarbamate. Flammable gases are generated by the combination of thiocarbamates and dithiocarbamates with aldehydes, nitrides, and hydrides. Thiocarbamates and dithiocarbamates are incompatible with acids, peroxides, and acid halides.Угроза здоровью
THIOFANOX is a carbamate pesticide. Carbamate pesticides are moderately to highly toxic. It is a cholinesterase inhibitor.Пожароопасность
(Non-Specific -- Carbamate Pesticide, Solid, n.o.s.) Container may explode in heat of fire. When heated to decomposition, THIOFANOX emits very toxic fumes of nitrogen and sulfur oxides. Stable at normal storage temperature; reasonably stable to hydrolysis at less than 86F at pH 5-9.Фармаколо?гия
The insecticidal carbamates are cholinergic. Poisoned insects and animals exhibit violent convulsions and other neuromuscular disturbances. These insecticides carbamylate acetylcholinesterase and may have a direct action on acetylcholine receptors. The mechanism of interaction with acetylcholinesterase is analogous to the normal three-step hydrolysis of acetylcholine. However, the third reaction step is much slower for the carbamylated enzyme than for the acetylated one. The importance of structural complementarity of the insecticidal carbamates to the active site of acetylcholinesterase is demonstrated by the pronounced difference in activities of D-2-(sec-butylphenyl) methylcarbamate and L-2-(sec-butylphenyl) methylcarbamate (the L isomer is five times more toxic) and of the 2-, 3-, and 4-substituted phenylmethylcarbamates, where the 4-isomers are virtually inactive.Detoxification of carbamate insecticides occurs in vivo through microsomal hydroxylation, N-demethylation of carbamyl nitrogen, side chain oxidation, and ring hydroxylation. Methylenedioxyphenyl synergists prevent oxidation
Возможный контакт
A potential danger to those involved in the manufacture, formulation and application of this thiocarbamate systemic insecticide and acaricide.Метаболический путь
The hydrolytic degradation and metabolism of thiofanox in soils, plants and animals follow a common pathway. Oxidation of the S-methyl moiety is the primary reaction to yield the thiofanox sulfoxide and sulfone. Hydrolysis of the carbamate linkage to yield the oximes is only a minor pathway for thiofanox and its oxidation products (Scheme 1).Перевозки
UN2757 Carbamate pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.Несовместимости
Thiocarbamate esters are combustible. They react violently with powerful oxidizers such as calcium hypochlorite. Poisonous gases are generated by the thermal decomposition of thiocarbamate compounds, including carbon disulfide, oxides of sulfur, oxides of nitrogen, hydrogen sulfide, ammonia, and methylamine. Many materials in this group slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids. Flammable gases are generated by the combination ofУтилизация отходов
In accordance with 40CFR 165 recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.