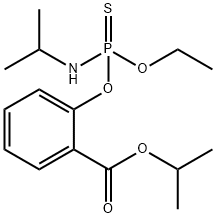
ISOFENPHOS
- русский язык имя
- английское имяISOFENPHOS
- CAS №25311-71-1
- CBNumberCB6667609
- ФормулаC15H24NO4PS
- мольный вес345.39
- EINECS246-814-1
- номер MDLMFCD00041805
- файл Mol25311-71-1.mol
| Температура плавления | -12℃ |
| Температура кипения | bp0.01 120° |
| плотность | 1.131 g/cm3 (20 ºC) |
| давление пара | 2.2 x 10-4 Pa (20 °C) |
| температура хранения | 0-6°C |
| растворимость | Chloroform (Slightly), DMSO (Slightly) |
| Растворимость в воде | 18 mg l-1 (20 °C) |
| пка | -0.30±0.70(Predicted) |
| форма | Oil |
| цвет | Colourless |
| Справочник по базе данных CAS | 25311-71-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 3 |
| FDA UNII | 0514MAW53A |
| Пестициды Закон о свободе информации (FOIA) | Isofenphos |
| Система регистрации веществ EPA | Isofenphos (25311-71-1) |
| Коды опасности | T,N,Xn,F |
| Заявления о рисках | 24/25-50/53-52/53-36-20/21/22-11 |
| Заявления о безопасности | 36/37-45-60-61-26-16 |
| РИДАДР | 3018 |
| WGK Германия | 2 |
| RTECS | VO4395500 |
| Класс опасности | 6.1(a) |
| Группа упаковки | I |
| кода HS | 29299090 |
| Банк данных об опасных веществах | 25311-71-1(Hazardous Substances Data) |
| Токсичность | LD50 orally in rats, mice (mg/kg): 30-40, 90-130 (Homeyer) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H302+H312+H332:Вредно при проглатывании, при попадании на кожу или при вдыхании.
H412:Вредно для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
ISOFENPHOS химические свойства, назначение, производство
Химические свойства
Isofenphos is a colorless oil at room temperature. It is sparingly soluble in water, but soluble in cyclohexone, toluene, acetone, and diethyl ether. The US EPA has grouped isofenphos under RUP, which indicates that qualifi ed, certifi ed, and trained workers are required in the safety management of isofenphos. It is used on turf and ornamental trees and shrubs to control white grubs, mole crickets, and other insects, such as soil-dwelling insects, cabbage root fl ies, corn roundworms, and wire worms.Использование
Isofenphos is an organophosphorus insecticide used in soil to control leaf-eating and soil-dwelling pests in vegetables, fruits, turf and field crops.Общее описание
Colorless oil. Non corrosive. Used as an insecticide.Реакции воздуха и воды
Hydrolyzed by alkali solution.Профиль реактивности
Organothiophosphates, such as ISOFENPHOS, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.Угроза здоровью
Exposures to isofenphos are highly toxic to animals and humans. Acute and prolonged exposures to high concentrations of isofenphos has caused poisoning with symptoms such as increased secretions, diffi culty in breathing, diarrhea, urination, pupil contraction, slowness of the heart, convulsions, and coma. Isofenphos in combination with malathion cause severe poisoning in humans.Экологическая судьба
Soil. Rapidly degraded by microbes via oxidative desulfuration in soils forming isofenphos oxon (Abou-Assaf et al., 1986; Abou-Assaf and Coats, 1987; Somasundarum et al., 1989), isopropyl salicylate and carbon dioxide (Somasundaram et al., 1989). The formation of isofenphos oxon is largely dependent upon the pH, moisture and temperature of the soil. The degradation rate of isofenphos decreased with a decrease in temperature (35°C >25°C >15°C), moisture content (22.5% >30% >15%) and in acidic and alkaline soils (pH 6 and 8 >pH 7). After isofenphos was applied to soil at a rate of 1,12 kg/ai/ha, concentrations of 8.3, 7.2, 5.1 and 1.0 ppm were found after 5, 21, 43 and 69 days, respectively. Following a second application, 4.9, 1.55, 0.25 and 0.10 ppm of isofenphos were found after 5, 21, 43 and 69 days, respectively (Abou-Assaf and Coats, 1987).A pure culture of Arthrobacter sp. was capable of degrading isofenphos at different soil concentrations (10, 50 and 100 ppm) in less than 6 hours. In previously treated soils,isofenphos could be mineralized to carbon dioxide by indigenous microorganisms (
Plant. Two weeks following application to thatch, 65% of the applied amount was present (Sears et al., 1987).
Surface Water. In estuarine water, the half-life of isofenphos ranged from 9.8 to 11.9 days (Lacorte et al., 1995).
Photolytic. Irradiation of an isofenphos (500 mg) in hexane and methanol (100 mL) using a high pressure mercury lamp (l = 254–360 nm) for 24 hours yielded the following products: isofenphos oxon and O-ethyl hydrogen-N-isopropylphosphoroamidothioa
Метаболический путь
14C-Isofenphos is bioactivated by mixed-function oxidases that ultimately give N-desisopropylisofenphos oxon, a product with 2300-fold greater inhibitory potency than isofenphos oxon towards housefly head acetylcholinesterase.271 By housefly and the cuperous chafer, isofenphos is metabolized to give detoxified metabolites without the bioactivated N- desisopropylisofenphos oxon. No difference in kinds of metabolite is found between the two insects. When rats are administered 14C-isofenphos, most of the radioactivity is recovered from the water fraction of the urine and feces. Aminoisofenphos and isofenphos oxon are detected in the benzene fraction, and the other six metabolites are identified as water-soluble metabolites which include O-ethylhydrogen phosphoramidate and O-ethylhydrogen isopropylphosphoramidothioate. Water-soluble metabolites are predominantly formed through cleavage of the P-O-aryl linkage.Метаболизм
Main degradation route is cleavage of the P?O-aryl ester linkage through oxidative desulfuration to isofenphos oxon followed by hydrolysis and oxidative dearylation from isofenphos. In plant, the major metabolites are salicylic acid and dihydroxybenzoic acid.