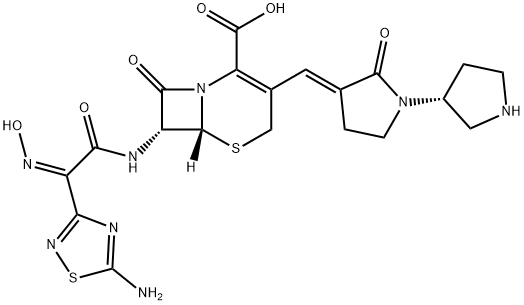
Цефтобипрол
- английское имяCeftobiprole
- CAS №209467-52-7
- CBNumberCB61509106
- ФормулаC20H22N8O6S2
- мольный вес534.57
- номер MDLMFCD09954116
- файл Mol209467-52-7.mol
химическое свойство
| плотность | 2.00±0.1 g/cm3(Predicted) |
| температура хранения | Store at -20°C,unstable in solution, ready to use. |
| растворимость | DMSO : 5 mg/mL (9.35 mM) |
| форма | Solid |
| пка | 2.46±0.50(Predicted) |
| цвет | Light yellow to yellow |
| FDA UNII | 5T97333YZK |
| UNSPSC Code | 12352200 |
| NACRES | NA.25 |
Цефтобипрол химические свойства, назначение, производство
Использование
Broad spectrum antibiotic.Антимикробная активность
The most important property distinguishing it from older cephalosporins is activity against methicillin-resistant staphylococci, a property conferred by a high affinity for penicillinbinding protein 2′ (2a). MICs for methicillin-resistant strains are nevertheless somewhat higher than those seen with fully susceptible strains. A similar situation exists with coagulasenegative staphylococci and with Str. pneumoniae, for which strains with reduced susceptibility to penicillin are less susceptible than fully resistant strains, while remaining within therapeutically achievable levels.Otherwise activity approximates to that of cephalosporins of group 4 . Activity against Ps. aeruginosa is modest and much reduced against ceftazidime-resistant strains.
Приобретенная устойчивость
It is hydrolyzed by extended spectrum β-lactamases of enterobacteria , which are therefore resistant. The prospects for the emergence of resistance during extensive clinical use are presently unclear, though increased resistance in Staph. aureus appears to be difficult to induce under laboratory conditions.Фармакокине?тика
Cmax 500 mg (667 mg prodrug): c. 35 mg/L end infusionintravenous (30-min infusion)
Plasma half-life: c. 3 h
Volume of distribution: 18.4 L
Plasma protein binding: 16%
The prodrug is rapidly hydrolyzed in plasma to release the active form together with diacetyl (2,3-butanediol) and CO2. Distribution approximates to the extracellular fluid volume in adults. There is no accumulation on repeat dosing in subjects with normal renal function.
It is chiefly excreted in urine by glomerular filtration. A urinary concentration exceeding 1 g/L is achieved within the first 2 h of a 500 mg (active drug equivalent) dose and 80–90% of active drug can be recovered within 24 h.
Клиническое использование
Ceftobiprole can be used as Broad spectrum antibiotic and in complicated infections of skin and skin structures.Побочные эффекты
Limited studies have so far revealed no unexpected side effects. Nausea, vomiting and altered taste sensation appear to be the most common.Цефтобипрол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-13131129325 | China | 5887 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +1-631-485-4226 | United States | 19553 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| 8485655694 | United States | 63687 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| United States | 24072 | 58 | ||
| United States | 38631 | 58 | ||
| +8618327326525 | China | 8467 | 58 | |
| +86-852-30606658 | China | 43340 | 58 |