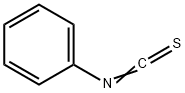
ФЕНИЛИЗОТИОЦИАНАТ
- английское имяPhenyl isothiocyanate
- CAS №103-72-0
- CBNumberCB5733806
- ФормулаC7H5NS
- мольный вес135.19
- EINECS203-138-1
- номер MDLMFCD00004798
- файл Mol103-72-0.mol
| Температура плавления | −21 °C(lit.) |
| Температура кипения | 218 °C(lit.) |
| плотность | 1.132 g/mL at 20 °C(lit.) |
| давление пара | 10 hPa (20 °C) |
| показатель преломления | n |
| Fp | 190 °F |
| температура хранения | 2-8°C |
| растворимость | water: insoluble |
| форма | liquid |
| цвет | Clear pale yellow to yellow |
| Удельный вес | 1.137 (20/4℃) |
| Растворимость в воде | Soluble in alcohol, and ether. Insoluble in water. |
| Чувствительный | Moisture Sensitive |
| Мерк | 14,7297 |
| БРН | 471392 |
| Диэлектрическая постоянная | 10.7(20℃) |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents, strong acids. |
| ИнЧИКей | QKFJKGMPGYROCL-UHFFFAOYSA-N |
| LogP | 3.280 |
| Surface tension | 41.5mN/m at 20°C |
| Справочник по базе данных CAS | 103-72-0(CAS DataBase Reference) |
| FDA UNII | 0D58F84LSU |
| Справочник по химии NIST | Benzene, isothiocyanato-(103-72-0) |
| Система регистрации веществ EPA | Benzene, isothiocyanato- (103-72-0) |
| UNSPSC Code | 12352209 |
| NACRES | NA.31 |
| Коды опасности | T,N,Xn,F | |||||||||
| Заявления о рисках | 25-34-42/43-51/53-67-65-50/53-36/37/38-22-11-38-63-23/24/25-36/38 | |||||||||
| Заявления о безопасности | 9-16-29-33-60-61-62-36-26-23-45-36/37/39-38-28A-36/37 | |||||||||
| РИДАДР | UN 1993 3/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | NX9275000 | |||||||||
| F | 19 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2930 90 98 | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | II | |||||||||
| Токсичность | LD50 orally in Rabbit: 157 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H334:При вдыхании может вызывать аллергическую реакцию (астму или затрудненное дыхание).
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
ФЕНИЛИЗОТИОЦИАНАТ химические свойства, назначение, производство
Химические свойства
colourless to pale yellow liquid with a penetrating odourИспользование
Phenyl isothiocyanate is the reagent of choice in automated Edman degradation systems. However, this reagent is highly toxic and for manual modification other reagents are preferred such as dimethy laminoazobenzene isothiocyanate (Chang, 1983; Wang et al, 2000) or trifluoroethyl isothiocyanate (Bartlet-Jones et al, 1994). This last reagent has the advantage of being volatile, so that the excess of reagent is easily removed by vacuum before MS analysis (Spengler, 1997). This compound was used successfully during seven successive cycles of manual cleavage coupled with MS analysis. Nevertheless, the high reactivity of such compounds seems to shows artefactual modification such as“acetylation' of the hydroxyl groups of the Ser and Thr. Allyl isothiocyanate shows better selectivity, but again this reagent is toxic and difficult to use in manual approaches (Gu & Preswich, 1997) .Определение
ChEBI: Phenyl isothiocyanate is an isothiocyanate having a phenyl group attached to the nitrogen; used for amino acid sequencing in the Edman degradation. It has a role as an allergen and a reagent.Подготовка
Synthetic procedure for phenyl isothiocyanate in 1-mol scaleInto a 2-L jacketed flask, 91.2 g of CS2 (1.2 mol) was dropwise added to a mixture of aniline (93.0 g, 1.0 mol) and K2CO3 (276.0 g, 2.0 mol) in 700 mL of water at room temperature within a period of 2.5 h. After the addition was complete, the mixture was stirred another 2 h. Then, the mixture was cooled to 0 °C, and a solution of 92.3 g of TCT (0.5 mol) in 450 mL of CH2Cl2 was dropwise added within 4 h. After the addition was complete, the mixture was stirred another 1 h to complete the conversion. The resulting mixture was basified to pH >11 with 250 mL of 6 N NaOH and yielded a clear solution. The organic layer was separated and the aqueous layer was extracted with 150 mL of CH2Cl2. The combined organic layers were dried over anhydrous Na2SO4, filtered, and the solvent of filtrate was removed via distillation under atmospheric pressure with a 25-cm Vigreux column. The residue was vacuum distilled and the desired product fraction was collected at 72–74 °C/1 mmHg. Finally, 127.0 g of colorless liquid (94%) was obtained.
Биологические функции
Phenyl isothiocyanate (PITC) is a well-established reagent in protein chemistry since its introduction in Edman degradation. PITC reacts with primary and secondary amines under alkaline conditions within 20 min. The resulting phenylthiocarbamyl (PTC) derivatives of the amino acids are stable and do not interfere with reaction by-products during chromatography. The absorption maximum is around 245 nm with a detection limit of 1 pmol.Phenyl isothiocyanate may be employed as a derivatization reagent for high-performance liquid chromatographic (HPLC) analysis of various amphetamine derivatives in body fluids for forensic purposes.
Общее описание
Phenyl isothiocyanate is an aromatic isothiocyanate. It participates in dehydration reactions of alcohols. It is widely used for synthesis of various biologically important heterocyclic compounds.Методы очистки
It is insoluble in H2O, but soluble in Et2O and EtOH. If impure (due to formation of thiourea), then steam distil it into a receiver containing 5-10mL of N H2SO4. Separate the oil, dry over CaCl2 and distil it under vacuum. [Dains et al. Org Synth Coll Vol I 447 1941, Beilstein 12 IV 867.]ФЕНИЛИЗОТИОЦИАНАТ запасные части и сырье
ФЕНИЛИЗОТИОЦИАНАТ поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +undefined18621330623 | China | 28 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12815 | 58 | ||
| +86-13131129325 | China | 5872 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29730 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21630 | 55 | ||
| +86-0371-86658258 +8613203830695 |
China | 29862 | 58 | ||
| +86 18953170293 | China | 2930 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 | ||
| 86-13657291602 | CHINA | 22963 | 58 | ||
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 |
ФЕНИЛИЗОТИОЦИАНАТ Обзор)
- 4-бромфенил изотиоциана
- Fluorescein 5(6)-isothiocyanate
- Гуанидинтиоцианата
- Флуоресцеин изотиоцианат изомер I
- Пропил изотиоциана
- Бензилизотиоцианата
- Изобутиловый изотиоциана
- Метилизотиоциана
- 1-бутил-изотиоциана
- Циклогексил изотиоциана
1of4