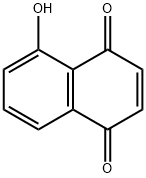
5-гидрокси-p-нафтохинон
- английское имя5-Hydroxy-1,4-naphthalenedione
- CAS №481-39-0
- CBNumberCB5447002
- ФормулаC10H6O3
- мольный вес174.15
- EINECS207-567-5
- номер MDLMFCD00001684
- файл Mol481-39-0.mol
химическое свойство
| Температура плавления | 161-163 °C (lit.) |
| Температура кипения | 265.11°C (rough estimate) |
| плотность | 1.2346 (rough estimate) |
| показатель преломления | 1.5036 (estimate) |
| температура хранения | Keep in dark place,Sealed in dry,Room Temperature |
| растворимость | DMSO: 10 mg/ml; Ethanol: 10 mg/ml |
| форма | Crystalline Powder |
| пка | 6.59±0.20(Predicted) |
| цвет | Orange to brown |
| Растворимость в воде | SOLUBLE IN HOT WATER |
| Чувствительный | Light Sensitive |
| Мерк | 14,5269 |
| БРН | 1909764 |
| LogP | 1.920 |
| Справочник по базе данных CAS | 481-39-0(CAS DataBase Reference) |
| FDA UNII | W6Q80SK9L6 |
| Справочник по химии NIST | 1,4-Naphthalenedione, 5-hydroxy-(481-39-0) |
| Система регистрации веществ EPA | 1,4-Naphthalenedione, 5-hydroxy- (481-39-0) |
| UNSPSC Code | 51111800 |
| NACRES | NA.77 |
больше
| Коды опасности | T | |||||||||
| Заявления о рисках | 25-36/37/38 | |||||||||
| Заявления о безопасности | 22-26-36/37/39-45-37/39-28A | |||||||||
| РИДАДР | UN 2811 6.1/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | QJ5775000 | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29146990 | |||||||||
| Токсичность | LD50 oral in rat: 112mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
5-гидрокси-p-нафтохинон химические свойства, назначение, производство
Описание
Juglone (5-hydroxynapthoquinone), is found in the leaves and other parts of walnut, hickory and pecan (1,2). Juglone is synthesized from isochorismic acid (a product of the shikimic acid pathway) and 2-oxo-glutaric acid (3). In plant tissue juglone exists as a free compound or as a glycoside (3,4). Action of a glucosidase releases 1,4,5-trihydroxynapthalene, which is then oxidized to juglone (3,4).Химические свойства
Orange to brown crystalline powderИспользование
antineoplastic, antifungal, antioxidant, Pin 1 inhibitorОпределение
ChEBI: A hydroxy-1,4-naphthoquinone that is 1,4-naphthoquinone in which the hydrogen at position 5 has been replaced by a hydroxy group.Общее описание
This substance is a primary reference substance with assigned absolute purity (considering chromatographic purity, water, residual solvents, inorganic impurities). The exact value can be found on the certificate. Produced by PhytoLab GmbH & Co. KGФармаколо?гия
Juglone is probably best known as the allelochemical produced by black walnut. The glucoside of juglone leaches from the leaves and branches of black walnut, where it is converted to juglone in the soil. Juglone is toxic to certain plant species and also inhibits the germination of seeds (4). Thus its allelopathic activity may be the result of both phytotoxicity and a germination inhibitor. Juglone is also antifungal (1,5) and attempts to correlate its presence with disease resistance in pecan, black walnut, and hickory to several fungal pathogens have been reported (1,2,5,6). Positive correlations have been found for resistance of juvenile leaves of black walnut to anthracnose caused by Gnomia leptostyla (5) and of some Carya species to the scab pathogen Cladosporium carygenum (2). In some pecans (C. illinoensis), juglone may act as both a preformed and an induced defense factor because concentrations of juglone increase after infection by fungi (2). No correlation between juglone glycoside concentration in pecan leaves and resistance pecan to C. carygenum has been reported (6). Free juglone and the glycosides increase after infection, but these increases could not be correlated with scab resistance (6).Методы очистки
Crystallise Juglone from *benzene/pet ether or pet ether. [Beilstein 8 III 2558, 8 IV 2368.]5-гидрокси-p-нафтохинон запасные части и сырье
5-гидрокси-p-нафтохинон поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8618829768577 | China | 1249 | 58 | ||
| +86-0533-2185556 +8617865335152 |
China | 10986 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 18017610038 | CHINA | 3619 | 58 | ||
| 18283602253; +8618283602253 |
China | 953 | 58 | ||
| 028-87075086 13350802083 |
CHINA | 1824 | 58 | ||
| +86-755-89396905 +86-15013857715 |
China | 10453 | 58 | ||
| +86-28-82633860; +8618080483897 |
China | 3772 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 |