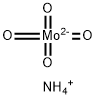
Молибдат аммония
- английское имяAmmonium molybdate
- CAS №13106-76-8
- CBNumberCB5309999
- ФормулаH4MoNO4-
- мольный вес177.99
- EINECS236-031-3
- номер MDLMFCD00011596
- файл Mol13106-76-8.mol
химическое свойство
| плотность | 2.498 |
| температура хранения | Inert atmosphere,Room Temperature |
| растворимость | ethanol: insoluble(lit.) |
| форма | Powder |
| цвет | White |
| Растворимость в воде | Soluble in water, acid and alkali, but insoluble in alcohol. |
| Пределы воздействия | ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 1000 mg/m3 |
| Справочник по базе данных CAS | 13106-76-8(CAS DataBase Reference) |
| Система регистрации веществ EPA | Molybdate (MoO42-), ammonium (1:2), (T-4)- (13106-76-8) |
| UNSPSC Code | 12352302 |
| NACRES | NA.23 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 26-36 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | QA4900000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 28417010 | |||||||||
| Токсичность | LD50 orl-rat: 333 mg/kg 28ZLA8 -,214,61 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Молибдат аммония химические свойства, назначение, производство
Химические свойства
Ammonium molybdate is a colorless, white or slightly greenish-yellow powder. It is used in chemical analysis for the determination of phosphorus. From a nitric acid solution it precipitates phosphorus in the form of ammonium phosphomolybdate having the formula (NH4)3PO4-12MoO3 after drying at 110 °C(230°F). Some of the phosphomolybdic acids are used as reagents for the alkaloids and in the analysis and separation of the alkali metals.Использование
Ammonium molybdate has been used to prepare phosphomolybdates. (NH4)2MoO4 was used as an analytical reagent for quantitative analysis of phosphates, silicates, arsenates and lead in their aqueous solutions. At saturated concentration, (NH4)2MoO4 was used to perform cryo-negative staining and as a negative stain in biological electron microscopy. Ammonium molybdate was used in study of crystallisation of cell-free-expressed membrane proteins.Методы производства
Pure molybdic oxide (99.95% MoO3) is prepared by sublimation of technical oxide or by calcining ammonium molybdate. Metallic molybdenum powder is prepared commercially by hydrogen reduction of either pure molybdic oxide or ammonium molybdate in a two-step process.Определение
ChEBI: Ammonium molybdate is an ammonium salt composed of ammonium and molybdate ions in a 2:1 ratio. It has a role as a poison. It contains a molybdate.Общее описание
Ammonium molybdate can be prepared by dissolving molybdenum trioxide in ammonia.Профиль безопасности
Poison by ingestion and intraperitoneal routes. Moderately toxic by other routes. An irritant. See also MOLYBDENUM COMPOUNDS. When heated to decomposition it emits toxic fumes of NH3 and NOx.Возможный контакт
It is used as an analytical reagent, in pigments and in the production of molybdenum metal and ceramics.Перевозки
There are no known UN/DOT restrictions.Методы очистки
Crystallise the salt from water (2.5mL/g) by partial evaporation in a desiccator. [Sturdivant J Am Chem Soc 59 630 1937, Grüttner & Jauder in Handbook of Preparative Inorganic Chem (Ed. Brauer) Academic Press Vol II p 1711 1965.]Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides; chemically active metals (such as potassium, sodium, magnesium and zinc) may cause a violent reaction.Молибдат аммония запасные части и сырье
Молибдат аммония поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 |