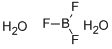
Бора трифторид дигидра
- английское имяBoron trifluoride dihydrate
- CAS №13319-75-0
- CBNumberCB4853046
- ФормулаBF3H4O2
- мольный вес103.84
- EINECS603-716-7
- номер MDLMFCD00149901
- файл Mol13319-75-0.mol
химическое свойство
| Температура плавления | 6 °C |
| Температура кипения | 58-60 °C (1.5 mmHg) |
| плотность | 1.636 g/mL at 25 °C(lit.) |
| показатель преломления | n |
| форма | Liquid |
| цвет | Clear colorless to pale yellow |
| Растворимость в воде | miscible |
| Рейтинг продуктов питания EWG | 1 |
| Система регистрации веществ EPA | Borane, trifluoro-, dihydrate (13319-75-0) |
| UNSPSC Code | 12352106 |
| NACRES | NA.22 |
| Коды опасности | C,T | |||||||||
| Заявления о рисках | 34-48/23-35-20/22 | |||||||||
| Заявления о безопасности | 26-27-36/37/39-45-28A-23-28-9 | |||||||||
| РИДАДР | UN 2851 8/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | ED2285000 | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28261990 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H372:Поражает органы в результате многократного или продолжительного воздействия.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H330:Смертельно при вдыхании.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
Бора трифторид дигидра химические свойства, назначение, производство
Химические свойства
colourless liquidОбщее описание
Boron trifluoride dihydrate is a fuming liquid. Boron trifluoride dihydrate may be corrosive to skin, eyes and mucous membranes. Boron trifluoride dihydrate may be toxic by inhalation. Boron trifluoride dihydrate is used as a catalyst in chemical reactions.Реакции воздуха и воды
In moist air forms dense white fumes pungent, corrosive to skin, avoid inhalation [Merck 11th ed. 1989].Профиль реактивности
BORON TRIFLUORIDE is a colorless, strongly irritating, liquid. Upon contact with water, steam or when heated to decomposition, Boron trifluoride dihydrate will produce toxic fluoride fumes. Incompatible with alkyl nitrates, calcium oxide. Reaction with alkali metals or alkaline earth metals (except magnesium) will cause incandescence [Bretherick, 5th ed., 1995, p. 65].Угроза здоровью
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.Возможный контакт
Boron trifluoride is a highly reactive chemical used primarily as a catalyst in chemical synthesis. It is stored and transported as a gas, but can be reacted with a variety of materials to form both liquid and solid compounds. The magnesium industry utilizes the fireretardant and antioxidant properties of boron trifluoride in casing and heat treating. Nuclear applications of boron trifluoride include neutron detector instruments; boron-10 enrichment and the production of neutroabsorbing salts for molten-salt breeder reactors.Перевозки
UN1008 Boron trifluoride, Hazard class: 2.3; Labels: 2.3—Poisonous gas, 8—Corrosive material, Inhalation Hazard Zone B. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.Несовместимости
Boron trifluoride reacts with polymerized unsaturated compounds. Decomposes on contact with water, moist air, and other forms of moisture, forming toxic and corrosive hydrogen fluoride, fluoroboric acid, and boric acid. Reacts violently with alkali and alkaline earth metals (except magnesium); metals, such as sodium, potassium, and calcium oxide, and with alkyl nitrates. Attacks many metals in presence of water.Утилизация отходов
Return refillable compressed gas cylinders to supplier. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. Chemical reaction with water to form boric acid, and fluoroboric acid. The fluoroboric acid is reacted with limestone, forming boric acid and calcium fluoride. The boric acid may be discharged into a sanitary sewer system while the calcium fluoride may be recovered or landfilled. Protect cylinder and labels from physical damage.Бора трифторид дигидра Обзор)
- Нитрид бора
- Диэтилэфирата трехфтористого бора
- Нитрозоний тетрафторборат
- N-Ethylbenzisoxazolium Тетрафторбора
- Нитроний тетрафторборат
1of3