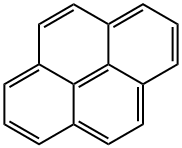
Пирен
- английское имяPyrene
- CAS №129-00-0
- CBNumberCB4853011
- ФормулаC16H10
- мольный вес202.25
- EINECS204-927-3
- номер MDLMFCD00004136
- файл Mol129-00-0.mol
химическое свойство
| Температура плавления | 148 °C |
| Температура кипения | 393 °C |
| плотность | 1.271 |
| Плотность накопления | 650kg/m3 |
| давление пара | 180 at 25 °C (extrapolated from vapor pressures determined at higher temperatures, Tesconi andYalkowsky, 1998) |
| показатель преломления | 1.8520 (estimate) |
| Fp | 210 °C |
| температура хранения | room temp |
| растворимость | ethanol: soluble |
| форма | crystalline |
| пка | >15 (Christensen et al., 1975) |
| цвет | Pale yellow to yellow-greenish |
| Удельный вес | 1.271 |
| Растворимость в воде | almost insoluble |
| λмакс | 330nm(EtOH)(lit.) |
| Мерк | 14,7963 |
| БРН | 1307225 |
| констант закона Генри | 0.490 at 25 °C (thermodynamic method-GC/UV spectrophotometry, Altschuh et al., 1999) |
| Пределы воздействия | OSHA: TWA 0.2 mg/m3 |
| ИнЧИКей | BBEAQIROQSPTKN-UHFFFAOYSA-N |
| LogP | 5.43 at 30℃ and pH5-8 |
| Справочник по базе данных CAS | 129-00-0(CAS DataBase Reference) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | PYRENE |
| Рейтинг продуктов питания EWG | 2 |
| FDA UNII | 9E0T7WFW93 |
| Справочник по химии NIST | Pyrene(129-00-0) |
| МАИР | 3 (Vol. Sup 7, 92) 2010 |
| Система регистрации веществ EPA | Pyrene (129-00-0) |
| UNSPSC Code | 41116107 |
| NACRES | NA.32 |
больше
| Коды опасности | N,T+,T,F,Xn | |||||||||
| Заявления о рисках | 50/53-36/37/38-26-39/23/24/25-23/24/25-11-63-43-45-67-65-38-51/53-52/53-40 | |||||||||
| Заявления о безопасности | 60-61-45-36/37-28A-22-16-7-24/25-23-53-62-26 | |||||||||
| РИДАДР | UN 3077 9/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | UR2450000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29349990 | |||||||||
| Банк данных об опасных веществах | 129-00-0(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 2700 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P391:Ликвидировать просыпания/проливы/утечки.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Пирен химические свойства, назначение, производство
Химические свойства
Pyrene is a colorless crystalline solid when pure or pale yellow plates (impure). Polycyclic aromatic hydrocarbons (PAHs) are compounds containing multiple benzene rings and are also called polynuclear aromatic hydrocarbons. Solids and solutions have a Blue fluores- cence (Merck Index).Физические свойства
Colorless solid (tetracene impurities impart a yellow color) or monoclinic prisms crystallized from alcohol. Solutions have a slight blue fluorescence.Использование
Pyrene occurs in coal tar. Also obtained by the destructive hydrogenation of hard coal. Found in wastewater in aquatic environments, and possesses genotoxic characteristics relating to estrogenic/andr ogenic, antiestrogenic and antiandrogenic activity.Методы производства
There is no commercial production or known use for this compound. Pyrene has been used as starting material for the synthesis of benzo[a]pyrene.Определение
A condensed ring hydro- carbon.Общее описание
Colorless solid, solid and solutions have a slight blue fluorescence. Used in biochemical research.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
Pyrene reacts with nitrogen oxides to form nitro derivatives. Pyrene also reacts with 70% nitric acid.Опасность
A questionable carcinogen, absorbed by skin.Угроза здоровью
Pyrene is a carcinogenic agent and is absorbed by the skin. It is a skin irritant, a suspected mutagen, and an equivocal tumor-causing agent. Workers exposed to 3 to 5 mg/m3 of Pyrene exhibited some teratogenic effects. Pyrene is a polycyclic aromatic hydrocarbon (PAH). The acute toxicity of pure PAHs appears low when administered orally or dermally to rats or mice. Human exposure to PAHs is almost exclusively via the gastrointestinal and respiratory tracts, and approximately 99 percent is ingested in the diet. Despite the high concentrations of Pyrene to which humans may be exposed through food, there is currently little information available to implicate diet-derived PAHs as the cause of serious health effects.Пожароопасность
When heated to decomposition, Pyrene emits acrid smoke and fumes.Профиль безопасности
Poison by inhalation. Moderately toxic by ingestion and intraperitoneal routes. A sh irritant. Questionable carcinogen with experimental tumorigenic data. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.Возможный контакт
Pyrene is used as an industrial chemi- cal and in biochemical research.Канцерогенность
Pyrene was inactive as a tumor initiator. Based on inadequate animal data and no human data, the IARC classified pyrene as group 3, not classifiable as to its carcinogenicity to humans, and IRIS classified pyrene as a class D compound. Pyrene is present as a major component of the total content of PAHs in the environment. Exposure occurs primarily through tobacco smoke, inhalation of polluted air, and by ingestion of food and water.Перевозки
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous haz- ardous material, Technical Name Required.Методы очистки
Crystallise pyrene from EtOH, glacial acetic acid, *benzene or toluene. Purify it also by chromatography of CCl4 solutions on alumina, with *benzene or n-hexane as eluent. [Backer & Whitten J Phys Chem 91 865 1987.] It can also be zone refined and purified by sublimation. Marvel and Anderson [J Am Chem Soc 76 5434 1954] refluxed pyrene (35g) in toluene (400mL) with maleic anhydride (5g) for 4days, then added 150mL of aqueous 5% KOH and refluxed for 5hours with occasional shaking. The toluene layer was separated, washed thoroughly with H2O, concentrated to about 100mL and allowed to cool. Crystalline pyrene was filtered off and recrystallised three times from EtOH or acetonitrile. [Chu & Thomas J Am Chem Soc 108 6270 1986, Russell et al. Anal Chem 50 2961 1986.] The material is free from anthracene derivatives. Another purification step involves passage of pyrene in cyclohexane through a column of silica gel. It can be sublimed in a vacuum and zone refined. The picrate has m 224o. [Kano et al. J Phys Chem 89 3748 1985, Beilstein 5 IV 2467.]Несовместимости
Pyrene Dust may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Pyrene reacts with nitrogen oxides to form nitro derivatives. It also reacts with 70% nitric acidУтилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed .Пирен запасные части и сырье
Пирен поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 571-88938639 +8617705817739 |
China | 52849 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +8613343047651 | China | 3692 | 58 | ||
| +8615350851019 | China | 1001 | 58 | ||
| +86-021-62885108 +8613917661608 |
China | 2068 | 57 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +undefined-21-51877795 | China | 32965 | 60 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| +86 18953170293 | China | 2930 | 58 |