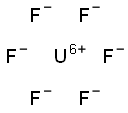
ГЕКСАФТОРИД УРАНА
- английское имяUranium hexafluoride
- CAS №7783-81-5
- CBNumberCB4852261
- ФормулаF6U
- мольный вес352.0193292
- EINECS232-028-6
- файл Mol7783-81-5.mol
химическое свойство
| Температура плавления | 64.8° |
| Температура кипения | 56.5°C (estimate) |
| плотность | d420.7 5.09; d70 (liq) 3.595 |
| растворимость | reacts with H2O; soluble in ctc, chloroform |
| форма | white monoclinic solid |
| цвет | white monoclinic solid |
| Растворимость в воде | soluble liquid Cl2, Br2; gives dark red fuming solution with nitrobenzene; soluble CCl4, CH3Cl [MER06] |
| FDA UNII | N06GJ1D94J |
| Система регистрации веществ EPA | Uranium fluoride (UF6), (OC-6-11)- (7783-81-5) |
| РИДАДР | 2978 |
| Класс опасности | 7 |
| Группа упаковки | II |
| Банк данных об опасных веществах | 7783-81-5(Hazardous Substances Data) |
ГЕКСАФТОРИД УРАНА химические свойства, назначение, производство
Описание
Uranium hexafluoride has a molecular formula of UF6. It is a colorless, volatile crystal that sublimes and reacts vigorously with water. It is highly corrosive and is a radiation risk. The four-digit UN identification number for fissile material containing more than 1% of uranium 235 is 2977; for lower specific activity, the number is 2978. Uranium hexafluoride is used in a gaseous diffusion process for separating isotopes of uranium.Химические свойства
Colorless, volatile crystals. Sublimes, triple point 64.0C (1134mmHg). Soluble in liquid bromine, chlorine, carbon tetrachloride, sym-tetrachloroethane, and fluorocarbons. Reacts vigorously with water, alcohol, ether, and most metals. Vapor behaves as nearly perfect gas.Физические свойства
White monoclinic crystals; density 5.09 g/cm3; melts at 64°C (triple point); sublimes at 56.6°C; critical temperature 232.65°C; critical pressure 46 atm; critical volume 250 cm3/mol; reacts with water forming UO2F2 and HF; soluble in chloroform, carbon tetrachloride and fluorocarbon solvents; soluble in liquid chlorine and bromine; dissolves in nitrobenzene to form a dark red solution that fumes in air.Использование
Uranium hexafluoride is used in uranium processing because its unique properties make it very convenient. It can conveniently be used as a gas for processing, as a liquid for filling or emptying containers or equipment, and as a solid for storage, all at temperatures and pressures commonly used in industrial processes.Определение
A crystalline volatile compound, used in separating uranium isotopes by differences in the rates of gas diffusion.Подготовка
There are many ways of making UF6. Although reaction of uranium and fluorine was first noted by Henri Moissan, first isolator of fluorine, c.1900, UF6 was originally reported by Ruff (pioneer of syntheses of many metal fluorides) and Heinzelmann in 1911. They made it by fluorination of uranium, and also by fluorination of UC2; fluorine needs to be heated to react with uranium, unless it is finely divided.U (s) + 3 F2 (g)---> UF6 (g)
UC2 (s) + 7 F2 (g) ---> UF6 (g) + 2 CF4 (g) (at 350°C)
An unusual reaction which does not use fluorine (but which has not been employed commercially) is:-
2 UF4 (g) + O2 (g)---> UF6 (g) + UO2F2 (g)
The main process used industrially employs fluorination of UF4 at around 500°C. It is a very exothermic process; temperatures can reach 1100°C in reactors with capacities of up to 380 kg per hour.
UF4 (g) + F2 (g)---> UF6 (g)
Общее описание
A colorless volatile radioactive crystalline solid. Highly toxic and corrosive. Radioactive. Emits high energy rays which may be harmful and are detectable only by special instruments. Chemically irritates skin, eyes and mucous membranes. Used to make fuel for nuclear power plants.Реакции воздуха и воды
Reacts vigorously with water to form uranyl fluoride (UO2F2) and corrosive hydrogen fluoride (hydrofluoric acid).Профиль реактивности
URANIUM HEXAFLUORIDE in which the uranium has been depleted of the isotope U-235. Naturally occurring uranium contains 0.7% U-235 and 99.3% U-238 (lower radioactivity). Thus, a depleted uranium material with some U-235 removed by the enrichment process is less radioactive. Emits fumes of highly toxic metallic uranium and uranium fluorides when heated to decomposition [Lewis, 3rd ed., 1993, p. 1301]. Reacts vigorously with aromatic hydrocarbons (benzene, toluene, xylenes), undergoes a violent reaction with water or alcohols (methanol, ethanol) [Bretherick, 5th ed., 1995, p. 1439]. Reacts with most metals.Опасность
Uranium hexafluoride is a corrosive substance and also presents radiation hazard.Угроза здоровью
Radiation presents minimal risk to transport workers, emergency response personnel and the public during transportation accidents. Packaging durability increases as potential radiation and criticality hazards of the content increase. Chemical hazard greatly exceeds radiation hazard. Substance reacts with water and water vapor in air to form toxic and corrosive hydrogen fluoride gas and an extremely irritating and corrosive, white-colored, water-soluble residue. If inhaled, may be fatal. Direct contact causes burns to skin, eyes, and respiratory tract. Low-level radioactive material; very low radiation hazard to people. Runoff from control of cargo fire may cause low-level pollution.Пожароопасность
Substance does not burn. The material may react violently with fuels. Containers in protective overpacks (horizontal cylindrical shape with short legs for tie-downs), are identified with "AF", "B(U)F" or "H(U)" on shipping papers or by markings on the overpacks. They are designed and evaluated to withstand severe conditions including total engulfment in flames at temperatures of 800°C (1475°F) for a period of 30 minutes. Bare filled cylinders, identified with UN2978 as part of the marking (may also be marked H(U) or H(M)), may rupture in heat of engulfing fire; bare empty (except for residue) cylinders will not rupture in fires. Radioactivity does not change flammability or other properties of materials.Профиль безопасности
Radioactive poison. A corrosive irritant to skin, eyes, and mucous membranes. Violent reaction with hydroxy compounds (e.g., ethanol, water). Vigorous reaction with aromatic hydrocarbons (e.g., benzene, toluene, xylene). When heated to decomposition it emits toxic fumes of F-. See also FLUORIDES and URANIUM.Методы очистки
Purify uranium hexafluoride by fractional distillation to remove HF. Also purify it by low-temperature trap-to-trap distillation over pre-dried NaF [Anderson & Winfield J Chem Soc, Dalton Trans 337 1986].ГЕКСАФТОРИД УРАНА запасные части и сырье
запасной предмет
ГЕКСАФТОРИД УРАНА поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| 029-029-81148696 18161915376 |
China | 9997 | 58 |