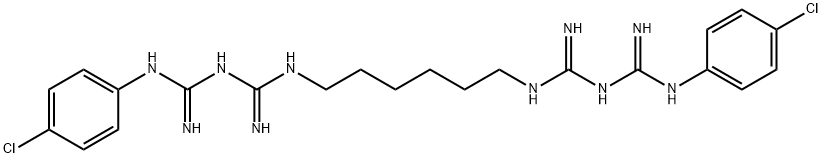
Хлоргексидин
- английское имяChlorhexidine
- CAS №55-56-1
- CBNumberCB4732251
- ФормулаC22H30Cl2N10
- мольный вес505.45
- EINECS200-238-7
- номер MDLMFCD00009673
- файл Mol55-56-1.mol
| Температура плавления | 134-136 °C (lit.) |
| Температура кипения | 641.45°C (rough estimate) |
| плотность | 1.1555 (rough estimate) |
| показатель преломления | 1.6300 (estimate) |
| температура хранения | 2-8°C |
| растворимость | water: soluble0.08% at 20°C |
| пка | pKa 10.78 (Uncertain) |
| форма | Solid |
| цвет | White to off-white |
| Растворимость в воде | 0.08 g/100 mL (20 ºC) |
| Мерк | 13,2108 |
| БРН | 2826432 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| ИнЧИКей | GHXZTYHSJHQHIJ-UHFFFAOYSA-N |
| LogP | 0.080 |
| FDA 21 CFR | 556.120 |
| Справочник по базе данных CAS | 55-56-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1-4 |
| FDA UNII | R4KO0DY52L |
| Словарь наркотиков NCI | chlorhexidine |
| Код УВД | A01AB03,B05CA02,D08AC02,D08AC52,D09AA12,R02AA05,S01AX09,S02AA09,S03AA04 |
| Система регистрации веществ EPA | Chlorhexidine (55-56-1) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xi,N |
| Заявления о рисках | 36/37/38-43-51/53-50/53 |
| Заявления о безопасности | 26-36/37-60-61 |
| РИДАДР | UN 3077 9/PG 3 |
| WGK Германия | 3 |
| RTECS | DU1925000 |
| F | 10-34 |
| Класс опасности | 9 |
| Группа упаковки | III |
| кода HS | 29215900 |
| Банк данных об опасных веществах | 55-56-1(Hazardous Substances Data) |
| Токсичность | LD50 orally in Rabbit: 5000 mg/kg |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H318:При попадании в глаза вызывает необратимые последствия.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P391:Ликвидировать просыпания/проливы/утечки.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Хлоргексидин химические свойства, назначение, производство
Описание
Chlorhexidine is a cationic broad-spectrum antimicrobial agent belonging to the bis(biguanide) family. Its mechanism of action involves destabilization of the outer bacterial membrane. It is effective on both Gram-positive and Gram-negative bacteria, although it is less effective with some Gram-negative bacteria. It has both bactericidal and bacteriostatic mechanisms of action.Chlorhexidine's antimicrobial effects are associated with the attractions between chlorhexidine (cation) and negatively charged bacterial cells. After chlorhexidine is absorpted onto the organism's cell wall, it disrupts the integrity of the cell membrane and causes the leakage of intracellular components of the organisms.
Aqueous solutions of chlorhexidine are most stable within the pH range of 5-8. Above pH 8.0 chlorhexidine base is precipitated and in more acid conditions there is gradual deterioration of activity because the compound is less stable. Chlorhexidine is used primarily as a topical antiseptic/disinfectant in wound healing, at catheterization sites, in various dental applications and in surgical scrubs. It has a LD-50 orally in mice as a diacetate at 2gm./kg. In digluconate form the LD-50 is 1800 gm./kg.
Химические свойства
Chlorhexidine occurs as an odorless, bitter tasting, white crystalline powder.Использование
Chlorhexidine is an antibacterial used for numerous applications. It is a cationic polybiguanide (bisbiguanide) used primarily as its salts, dihydrochloride, diacetate, and digluconate. Chlorhexidine is one of those drugs which are enlisted/included in the World Health Organization's List of Essential Medicines, a list of the most important drugs needed in a basic health system.- Chlorhexidine is used as a germicidal compound in teat dips. Also used as navel treatment, udder and eye wash, surgical scrub and sterilization material.
- Chlorhexidine is used primarily as a topical antiseptic/disinfectant in wound healing, at catheterization sites, in various dental applications and in surgical scrubs. it is used as an antibacterial agent in humans to control gingivitis and over all plaque control in preventative dentistry.
- Hydrogenolysis of benzyl-nitrogen bonds.
- Bacteriostatic;Detergent.
Методы производства
Chlorhexidine may be prepared either by condensation of polymethylene bisdicyandiamide with 4-chloroaniline hydrochloride or by condensation of 4-chlorophenyl dicyandiamine with hexamethylenediamine dihydrochloride. Chlorhexidine may also be synthesized from a series of biguanides.прикладной
Chlorhexidine is an important medical, dental and pharmaceutical antiseptic, disinfectant and preservative. It is bactericida and fungicidalsy but does not kill bacterial spores or mycobacteria, although it inhibits growth. It has a low order of activity against viruses, but high concentrations are effective in killing cysts of Acanthamoeba spp., organisms of potential clinical significance to the wearers of contact lenses. Properties Chlorhexidine is a bisbiguanide which is available as the acetate (diacetate), hydrochloride and gluconate salts. These are stable in solution and can be autoclaved although small amounts of chloroaniline are released.” As a cationic agent, chlorhexidine is incompatible with anionic surfactants and its antimicrobial activity is reduced in the presence of non-ionic surface-active agents. Activity is also reduced or abolished by phospholipids (a factor of significance in neutralizing chlorhexidine activity during the performance of biocidal tests) and by organic matter including serum. Some of these aspects have been well documented in the recent comprehensive paper of Nicoletti et al.” They also point out that the efficacy of chlorhexidine is influenced by formulation components and by the composition of the culture medium in which minimum inhibitory concentrations (MICs) are determined.Определение
ChEBI: A bisbiguanide compound with a structure consisting of two (p-chlorophenyl)guanide units linked by a hexamethylene bridge.Показания
This topical antiseptic product acts rapidly but, like hexachlorophene, persists on the skin to give a cumulative, continuing antibacterial effect. Like iodophors and alcohol, it is active against gram-positive and gram-negative bacteria, including P. aeruginosa, as well as common yeasts and fungi. It does not lose effectiveness in the presence of whole blood. Many consider it the antiseptic of choice for skin cleansing and surgical scrubs. Contact allergy is not uncommon. Chlorhexidine should not be used near the eyes or mucosal surfaces, because it may cause irritation or even anaphylaxis.Общее описание
Effect of the chlorhexidine:hydroxypropyl-β-cyclodextrin inclusion compound on in vitro slabs of bovine dentine has been investigated.Фармацевтические приложения
Chlorhexidine salts are widely used in pharmaceutical formulations in Europe and Japan for their antimicrobial properties. Although mainly used as disinfectants, chlorhexidine salts are also used as antimicrobial preservatives.As excipients, chlorhexidine salts are mainly used for the preservation of eye-drops at a concentration of 0.01% w/v; generally the acetate or gluconate salt is used for this purpose. Solutions containing 0.002–0.006% w/v chlorhexidine gluconate have also been used for the disinfection of hydrophilic contact lenses.
For skin disinfection, chlorhexidine has been formulated as a 0.5% w/v solution in 70% v/v ethanol and, in conjunction with detergents, as a 4% w/v surgical scrub. Chlorhexidine salts may also be used in topical antiseptic creams, mouthwashes, dental gels, and in urology for catheter sterilization and bladder irrigation.
Chlorhexidine salts have additionally been used as constituents of medicated dressings, dusting powders, sprays, and creams.
Клиническое использование
Chlorhexidine is a biguanide topical antiseptic and disinfectant with broad antimicrobial efficacy. It is increasingly being used as an aseptic but it is also gaining use as a biocidal ingredient in shampoos, conditioners, hair dyes, sunscreens, toothpastes, mouthwashes (Corsodyl), wet wipes (also for babies), eye creams, antiwrinkle creams, moisturizers, contact lens solutions, and instillation gels for urinary catheters.Urticaria following application to intact skin or mucosae, in some cases accompanied by dyspnea, angioedema, syncope, or anaphylaxis has been described via the mucosal route at much lower concentration than elsewhere, generally as low as 0.05%.Профиль безопасности
Poison by intraperitoneal andintravenous routes. Mildly toxic by ingestion.Experimental reproductive effects. A human skin irritant.Mutation data reported. When heated to decomposition itemits very toxic fumes of Cl- and NOx.Несовместимости
Chlorhexidine salts are cationic in solution and are therefore incompatible with soaps and other anionic materials. Chlorhexidine salts are compatible with most cationic and nonionic surfactants, but in high concentrations of surfactant chlorhexidine activity can be substantially reduced owing to micellar binding.Chlorhexidine salts of low aqueous solubility are formed and may precipitate from chlorhexidine solutions of concentration greater than 0.05% w/v, when in the presence of inorganic acids, certain organic acids, and salts (e.g. benzoates, bicarbonates, borates, carbonates, chlorides, citrates, iodides, nitrates, phosphates, and sulfates). At chlorhexidine concentrations below 0.01% w/v precipitation is less likely to occur.
In hard water, insoluble salts may form owing to interaction with calcium and magnesium cations. Solubility may be enhanced by the inclusion of surfactants such as cetrimide.
Other substances incompatible with chlorhexidine salts include viscous materials such as acacia, sodium alginate, sodium carboxymethylcellulose, starch, and tragacanth. Also incompatible are brilliant green, chloramphenicol, copper sulfate, fluorescein sodium, formaldehyde, silver nitrate, and zinc sulfate.
Interaction has been reported between chlorhexidine gluconate and the hydrogel poly(2-hydroxyethyl methacrylate), which is a component of some hydrophilic contact lenses.
Регуляторный статус
Chlorhexidine salts are included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.Хлоргексидин запасные части и сырье
Хлоргексидин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8617531153977 | China | 5855 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-18353166132 +86-18353166132 |
China | 983 | 58 | ||
| +8613343047651 | China | 3692 | 58 | ||
| +8613288715578 | China | 1174 | 58 | ||
| +86-86-02137122233 +8613795318958 |
China | 299 | 55 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 |