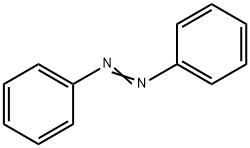
Азобензол
- английское имяAzobenzene
- CAS №103-33-3
- CBNumberCB4383484
- ФормулаC12H10N2
- мольный вес182.22
- EINECS203-102-5
- номер MDLMFCD00003022
- файл Mol103-33-3.mol
| Температура плавления | 65-68 °C (lit.) |
| Температура кипения | 293 °C (lit.) |
| плотность | 1.09 g/mL at 25 °C (lit.) |
| давление пара | 1 mm Hg ( 104 °C) |
| показатель преломления | 1.62662 (78.1℃) |
| Fp | 100 °C |
| температура хранения | room temp |
| растворимость | 6.4mg/l |
| форма | Crystalline Powder |
| цвет | Orange |
| Растворимость в воде | Soluble in alcohol, ether, benzene and glacial acetic acid. Insoluble in water. |
| Мерк | 14,917 |
| БРН | 1819138 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. Air and light sensitive. |
| Справочник по базе данных CAS | 103-33-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 4 |
| FDA UNII | F0U1H6UG5C |
| Предложение 65 Список | Azobenzene |
| МАИР | 3 (Vol. 8, Sup 7) 1987 |
| Система регистрации веществ EPA | Azobenzene (103-33-3) |
| UNSPSC Code | 12352124 |
| NACRES | NA.47 |
| Коды опасности | T,N,Xn,F | |||||||||
| Заявления о рисках | 45-20/22-48/22-50/53-68-67-65-62-51/53-38-11-48/20-39/23/24/25-23/24/25-52/53 | |||||||||
| Заявления о безопасности | 53-45-60-61-62-36/37-33-29-16-9-7 | |||||||||
| РИДАДР | UN 3077 9/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | CN1400000 | |||||||||
| Температура самовоспламенения | 890 °F | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29270000 | |||||||||
| Банк данных об опасных веществах | 103-33-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 1000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H350:Может вызывать раковые заболевания.
H302+H332:Вредно при проглатывании или при вдыхании.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P273:Избегать попадания в окружающую среду.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Азобензол химические свойства, назначение, производство
Описание
Azobenzene is a well-known derivative of stimulus-responsive molecular switches and has shown superior performance as a functional material in biomedical applications.Azobenzene is a typical photo-responsive molecule that isomerizes from its planar trans-form to the non-planar cis-form after UV-light irradiation with a wavelength between 300 nm and 400 nm (lmax is around 330 nm). Interestingly, the system reverts from the cis-form to the trans-form after further irradiation with visible light (wavelength over 400 nm). This process is completely reversible, and the azobenzene group does not decompose or induce undesirable side reactions even on repeated trans-cis isomerization. By introducing azobenzenes into DNA through D-threoninol as a linker, Asanuma and co-workers succeeded in achieving photo-regulation of:
Formation and dissociation of a DNA duplex
Transcription by T7-RNA polymerase reaction
Химические свойства
Azobenzene is a combustible, orange-red crystalline solid.Использование
acaricide, insecticide.Azobenzene can be used as an optical trigger for the design and synthesis of a large variety of photoresponsive systems.
Определение
ChEBI: A molecule whose structure comprises two phenyl rings linked by a N=N double bond; the parent compound of the azobenzene class of compounds.Общее описание
Orange-red crystals or dark brown chunky solid.Реакции воздуха и воды
AZOBENZENE is sensitive to air and light. Dust may form an explosive mixture in air. Insoluble in water.Профиль реактивности
AZOBENZENE is an azo compound. Azo, diazo, azido compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. AZOBENZENE is incompatible with strong oxidizing agents.Опасность
Toxic; may cause liver injury. Questionable carcinogen.Пожароопасность
Flash point data for AZOBENZENE are not available. AZOBENZENE is probably combustible.Профиль безопасности
Moderately toxic by ingestion and possibly other routes. Questionable carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. When heated to decomposition it emits toxic fumes of NOx.Возможный контакт
An azo compound. Those engaged in azobenzene use in dye, rubber, chemical, and pesticide manufacturing.Перевозки
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.Методы очистки
Ordinary azobenzene is nearly all in the trans-form. It is partly converted into the cis-form on exposure to light [for isolation see Hartley J Chem Soc 633 1938, and for spectra of cis-and trans-azobenzenes, see Winkel & Siebert Chem Ber 74B 6701941]. trans-Azobenzene is obtained by chromatography on alumina using 1:4 *C6H6/heptane or pet ether, and it crystallises from EtOH (after refluxing for several hours) or hexane. All operations should be carried out in diffuse red light or in the dark. [Beilstein 16 IV 8.]Несовместимости
Azo compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. This chemical is sensitive to prolonged exposure to heat. This chemical is incompatible with strong oxidizing agents.Азобензол запасные части и сырье
сырьё
запасной предмет
Азобензол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +undefined-21-51877795 | China | 32965 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 |