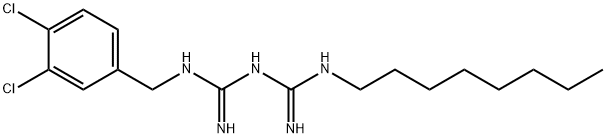
Оланексидин
- английское имяOLANEXIDINE
- CAS №146510-36-3
- CBNumberCB42451253
- ФормулаC17H27Cl2N5
- мольный вес372.34
- номер MDLMFCD09837698
- файл Mol146510-36-3.mol
химическое свойство
| Температура кипения | 454.7±55.0 °C(Predicted) |
| плотность | 1.22±0.1 g/cm3(Predicted) |
| пка | 11.92±0.10(Predicted) |
| FDA UNII | 92C2328G7P |
Оланексидин химические свойства, назначение, производство
Описание
In July 2015, olanexidine gluconate, a biguanide compound with remarkable antibacterial activity, was approved by the Pharmaceuticals and Medical Devices Agency (PMDA) of Japan for skin antisepsis at surgical sites. The drug was developed and marketed by Otsuka Pharmaceutical in Japan and is available as topical solution (1.5%). Olanexidine gluconate exhibited efficacy against a wide range of bacterial strains, especially Grampositive bacteria. In vitro experiments exploring its mechanism of action indicated that olanexidine interacts with bacterial surface molecules (such as lipopolysaccharides and lipoteichoic acid), disrupting the cell membranes of liposomes. These models suggest that the drug permeates the membranes of both Escherichia coli and Staphylococcus aureus and denatures proteins at relatively high concentrations (>160 g/mL).Синтез
The synthesis of olanexidine gluconate is relatively straightforward, involving the linkage of an n-octyl side chain and a dichlorobenzylamine through a bis-guanidyl lynchpin. The synthesis began with the reaction of commercial noctylamine (17) with sodium dicyanamide in the presence of concentrated sulfuric acid in refluxing n-butyl acetate to give rise to 1-cyano-3-octylguanidine (18) in 86% yield . Conditions employed to subsequently secure biguanidine 20 as the HCl salt hemihydrate in 77% yield were nearly identical to those used for the conversion of 17 to 18. Finally, treatment of 20 with sodium hydroxide in the presence of gluconic acid (21) gave rise to olanexidin gluconate (II) in almost quantitative yield.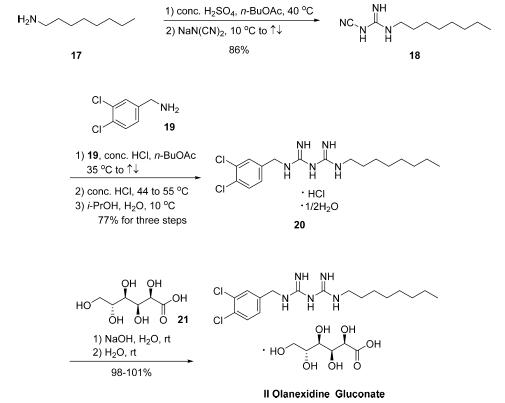
Оланексидин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 86-571-88216897,88216896 13588875226 |
CHINA | 6312 | 58 | |
| United States | 24072 | 58 | ||
| +86-0551-65418684 +8618949823763 |
China | 25356 | 58 | |
| +86-852-30606658 | China | 43340 | 58 | |
| +8613564774135 | United States | 19881 | 58 | |
| +8617320513646 | China | 9647 | 58 | |
| 400-6009262 16621234537 |
China | 14103 | 59 | |
| 010-56205725 | China | 12335 | 58 | |
| 15076683720 | China | 5960 | 55 | |
| 0371-037163312495,13303845143 13303845143 |
China | 10364 | 58 |