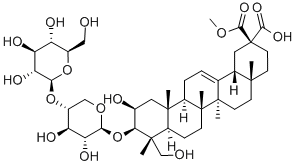
Esculentoside A
- русский язык имя
- английское имяEsculentoside A
- CAS №65497-07-6
- CBNumberCB3983421
- ФормулаC42H66O16
- мольный вес826.96
- номер MDLMFCD30488995
- файл Mol65497-07-6.mol
химическое свойство
| Температура плавления | >192°C (dec.) |
| Температура кипения | 936.7±65.0 °C(Predicted) |
| плотность | 1.42±0.1 g/cm3(Predicted) |
| температура хранения | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| растворимость | DMSO (Slightly), Methanol (Slightly) |
| пка | 4.39±0.70(Predicted) |
| форма | Solid |
| цвет | Pale Yellow |
| Стабильность | Hygroscopic |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Заявления о безопасности | 24/25 |
| кода HS | 29389090 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Esculentoside A химические свойства, назначение, производство
Использование
Esculentoside A is a saponin isolated from the root of Phytolacca esculenta. Modulates the immune response. and effects cell proliferation and apoptosis in cells. Anti-inflammatory. 500Фармакокине?тика
Esculentoside A is a triterpenoid saponin isolated from the root of Phytolacca esculenta. Studies have revealed that this triterpenoid exhibits a strong anti-inflammatory property. Ci et al. (2015) observed the anti-inflammatory potency of esculentoside A against allergic airway inflammation in a mouse model. They found that the drug enhanced nuclear Nrf-2 translocation in the lungs of ovalbumin-challenged mice and also decreased airway inflammation (Ci et al., 2015). Esculentoside A enhanced the intracellular anti-oxidant potentials, reduced Th2 cytokine level and down-regulated the expression of adhesion molecule mRNA in lung tissues. Zhang et al. (2014) found that esculentoside A could rectify carbon tetrachlorideinduced acute liver injury in mice. The drug successfully cured the histopathological damage induced by carbon tetrachloride. Rectification has been also noted through the down-regulation of inflammatory molecules and oxidative stresses associated with PPAR-γ, NF-κB and ERK signal pathways (Zhang et al., 2014). Ma et al. (2013) analyzed the role of esculentoside A on lupus nephritis-induced BXSB mice (where male SB/Le mice and female C57BL/6 mice were hybridized, creating a recombinant inbred species). They observed that esculentoside A application significantly reduced urine protein excretion and improved renal function by promoting apoptosis of glomerular intrinsic cells and renal tubular epithelial cells (Ma et al., 2013). The efficacy was also aided by the down-regulation of inflammatory cytokines.Wang et al. (2016) elucidated the role of esculentoside A against acetaminophen-induced liver toxicity in mice and observed that Nrf2-regulated the survival mechanism via the AMPK/ Akt/GSK3β pathway.
Esculentoside A поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18017610038 | CHINA | 3619 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86-28-82633860; +8618080483897 |
China | 3772 | 58 | |
| 18905173768 | CHINA | 2972 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +1-631-485-4226 | United States | 19553 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| 18502101150 | CHINA | 1923 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-27-8439 4403 18971486879 |
CHINA | 2935 | 58 |