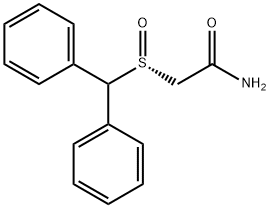
Армодафинил
- английское имяArmodafinil
- CAS №112111-43-0
- CBNumberCB3500844
- ФормулаC15H15NO2S
- мольный вес273.35
- номер MDLMFCD09841055
- файл Mol112111-43-0.mol
химическое свойство
| Температура плавления | 156-1580C |
| Температура кипения | 559.1±50.0 °C(Predicted) |
| плотность | 1.283 |
| температура хранения | Controlled Substance, -20°C Freezer |
| растворимость | DMSO: ≥16mg/mL |
| пка | 14.88±0.40(Predicted) |
| форма | powder |
| цвет | white to tan |
| оптическая активность | [α]/D -15 to -20° in methanol (C=1) |
| Словарь онкологических терминов NCI | armodafinil; Nuvigil |
| FDA UNII | V63XWA605I |
| Словарь наркотиков NCI | armodafinil |
| Код УВД | N06BA13 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 41 | |||||||||
| Заявления о безопасности | 26-39 | |||||||||
| WGK Германия | 3 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
H361d:Предполагается, что данное вещество может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Армодафинил химические свойства, назначение, производство
Описание
Armodafinil, an α1-adrenoceptor agonist, was launched for the oral treatment of excessive sleepiness associated with narcolepsy, SWSD, and obstructive sleep apnea/hypopnea syndrome (OSA). It is the R-enantiomer of modafinil, which is a previously marketed wake-promoting agent. The key differentiator for armodafinil is its longer pharmacokinetic half-life as compared with the S-enantiomer (10-14 hvs.3-4h). At therapeutic concentrations, armodafinil does not bind to most of the potentially relevant receptors for sleep/wake regulation (e.g., serotonin, dopamine, and adenosine receptors) or transporters of neurotransmitters or enzymes involved in sleep/wake regulation (e.g., serotonin, norepinephrine, and phosphodiesterase VI transporters). Both armodafinil and modafinil block dopamine reuptake by binding to the dopamine transporter and increasing dopamine concentrations in certain regions of the brain. However, dopamine receptor antagonists (e.g., haloperidol) and dopamine synthesis inhibitors (e.g., α-methyl-p-tyrosine) do not block modafinil’s action.In addition to its wake-promoting effects and ability to increase locomotor activity in animals, modafinil produces psychoactive and euphoric effects, alterations in mood, perception, thinking, and feelings typical of other CNS stimulants in humans. Modafinil was also partially discriminated as stimulant-like. However, the potential for abuse and dependency appears to be lower for modafinil than amphetamine-like stimulants.The most common adverse events associated with armodafinil included headache, nausea, dizziness, and insomnia.
Химические свойства
White SolidИспользование
Used for treatment of excessive sleepiness, a1-adrenoceptor agonistОпределение
ChEBI: A 2-[(diphenylmethyl)sulfinyl]acetamide that has R configuration at the sulfur atom. Like its racemate, modafinil, it is used for the treatment of sleeping disorders such as narcolepsy, obstructive sleep apnoea, and shift-work sleep disord r. Peak concentration in the blood later occurs later following administration than with modafinil, so it is thought that armodafinil may be more effective than modafinil in treating people with excessive daytime sleepiness.