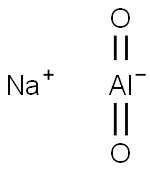
Sodium aluminate
- русский язык имя
- английское имяSodium aluminate
- CAS №11138-49-1
- CBNumberCB3332506
- ФормулаAlNaO2
- мольный вес81.97
- EINECS234-391-6
- номер MDLMFCD00085355
- файл Mol11138-49-1.mol
| плотность | 3.240 |
| форма | powder |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| Справочник по базе данных CAS | 11138-49-1(CAS DataBase Reference) |
| Специальный комитет по веществам GRAS | Sodium aluminate (packaging) Sodium phosphoaluminate (packaging) |
| FDA 21 CFR | 173.310; 182.90 |
| Рейтинг продуктов питания EWG | 3 |
| FDA UNII | 2VMT26903W |
| Система регистрации веществ EPA | Aluminum sodium oxide (11138-49-1) |
| UNSPSC Code | 12161600 |
| NACRES | NA.22 |
| Коды опасности | C |
| Заявления о рисках | 35 |
| Заявления о безопасности | 26-36/37/39-45 |
| РИДАДР | UN 3260 8/PG 3 |
| WGK Германия | 1 |
| RTECS | BD1600000 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H290:Может вызывать коррозию металлов.
-
оператор предупредительных мер
P234:Хранить только в оригинальной упаковке.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P330+P331:ПРИ ПРОГЛАТЫВАНИИ: Прополоскать рот. Не вызывать рвоту!
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Sodium aluminate химические свойства, назначение, производство
Химические свойства
Sodium aluminate is a white crystalline solid or solution.Использование
Sodium aluminate is used in the treatment of industrial and municipal water supplies. It is also an effective precipitant for soluble phosphate in sewage and is especially useful in wastewater having low alkalinity.Large quantities of sodium aluminate are used in papermaking where it improves sizing, filler retention,and pitch deposition.The addition of sodium aluminate to titanium dioxide paint pigment improves the nonchalking performance of outdoor paints.
Sodium aluminate is widely used in the preparation of alumina-based catalysts. Aluminosilicate can be prepared by impregnating silica gel with alumina obtained from sodium aluminate and aluminum sulfate. Reaction of sodium aluminate with silica or silicates has produced porous crystalline aluminosilicates which are useful as adsorbents and catalyst support materials, ie, molecular sieves.
Определение
sodium aluminate: white solid, NaAlO2 or Na2Al2O4, which is insolublein ethanol and soluble in water giving strongly alkaline solutions;m.p. 1800°C. It is manufactured by heating bauxite with sodium carbonate and extracting the residue with water, or it may be prepared in the laboratory by adding excess aluminium to hot concentrated sodium hydroxide. In solutionthe ion Al(OH)4- predominates.Sodium aluminate is used as amordant, in the production of zeolites,in effluent treatment, in glass manufacture, and in cleansing compounds.Подготовка
Small amounts of sodium aluminate are prepared in the lab by fusion of equimolar quantities of sodium carbonate and aluminum acetate, Al(C2H3O2)3, at 800°C. Other methods involve reaction of sodium hydroxide with amorphous alumina or aluminum metal.Commercial quantities of sodium aluminate are made from hydrated alumina, in the form of aluminum hydroxy oxide, AlO(OH), or aluminum hydroxide, Al(OH)3, a product of the Bayer process which is used to refine bauxite, the principal aluminum ore.Commercial grades of sodium aluminate are obtained by digestion of aluminum trihydroxide in aqueous caustic at atmospheric pressure and near the boiling temperature.
Реакции
Sodium aluminate, NaAlO2, white solid, (1) by reaction of aluminum hydroxide and NaOH solution, (2) by fusion of aluminum oxide and sodium carbonate, the solution reacts with CO2 to form aluminum hydroxide.Общее описание
Water solution of the white crystalline powder. Corrosive to metals and tissue.Реакции воздуха и воды
Sodium aluminate will dissolve in water and produce a strong corrosive alkaline solution. May generate heat when water is added.Профиль реактивности
SODIUM ALUMINATE generates a strong base in water; reacts violently with acids and corrosive to metals. Not compatible with copper, tin, zinc, aluminum, acids, phosphorus, or chlorocarbons.Опасность
(Solution) Strong irritant to tissue.Угроза здоровью
Material is caustic. Irritates skin, eyes, and gastrointestinal tract, causing redness of skin and eyes, burning sensation of mucous membranes.Пожароопасность
Behavior in Fire: Containers may burst when exposed to heat.Профиль безопасности
Moderate irritant to skin, eyes, and mucous membranes. A corrosive substance. When heated to decomposition it emits toxic fumes of NazO.Возможный контакт
Used in water and waste treatment; papermaking industry; in printing on fabrics; in the manufacture of pigments, milk glass, and soap; hardening building stone; sizing paper; as a water softener.Перевозки
UN2812 Sodium aluminate, solid, Hazard class: 8; Labels: 8-Corrosive material. UN1819 Sodium aluminate, solution, Hazard class: 8; Labels: 8-Corrosive material.Несовместимости
The aqueous solution is a strong base. Reacts violently with acid. Incompatible with organic anhydrides; isocyanates, alkylene oxides; epichlorohydrin, aldehydes, alcohols, glycols, caprolactum, chlorocarbons. Corrosive to metals; attacks copper, tin, aluminum, and zinc.Sodium aluminate запасные части и сырье
Sodium aluminate поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8617531153977 | China | 5855 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +undefined18602966907 | China | 997 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18017610038 | CHINA | 3619 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 |