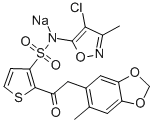
Sitaxentan sodium
- русский язык имя
- английское имяSitaxentan sodium
- CAS №210421-74-2
- CBNumberCB32459580
- ФормулаC18H14ClN2NaO6S2
- мольный вес476.89
- номер MDLMFCD11040990
- файл Mol210421-74-2.mol
химическое свойство
| температура хранения | room temp |
| растворимость | H2O: soluble10mg/mL (clear solution) |
| форма | powder |
| цвет | white to beige |
| Растворимость в воде | H2O: 10mg/mL (clear solution) |
| FDA UNII | 6V9JH46E20 |
| UNSPSC Code | 51111800 |
| NACRES | NA.77 |
Sitaxentan sodium химические свойства, назначение, производство
Описание
In November Encysive Pharmaceuticals launched Thelin® (sitaxsentan sodium) in the U.K. for the treatment of pulmonary arterial hypertension (PAH), following European Commission approval in August 2006. Sitaxsentan is the first selective endothelin A (ETA) receptor antagonist, and the first once-daily oral treatment available for patients with PAH. It is 6,500-fold selective in the targeting of ETA versus ETB receptors. Sitaxsentan is indicated for improving exercise capacity in PAH patients classified as World Health Organization (WHO) functional class III. Efficacy has been shown in primary pulmonary hypertension and pulmonary hypertension associated with connective tissue disease. In the U.S., Encysive has submitted a complete response to an approvable letter received from the FDA in July.Использование
Selective endothelin A (ETA) receptor antagonist. Antihypertensive. Used in treatment of chronic heart failure.Общее описание
Sitaxentan sodium, belongs to thesulfonamide class of endothelin receptor antagonists.Although it has 6,000-fold selectivity for the ETA receptor,clinical trials have not demonstrated a greater efficacyover bosentan. However, it has much lower liver toxicitythan bosentan. The manufacturer is attempting to meetefficacy outcomes set by the FDA, which must be met beforeapproval of sitaxsentan as a therapeutic agent will begranted.Sitaxentan sodium поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 0086-13720134139 | CHINA | 965 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-29-89586680 +86-15129568250 |
China | 22787 | 58 | |
| 571-88938639 +8617705817739 |
China | 52849 | 58 | |
| +1-708-310-1919 +1-13798911105 |
United States | 6391 | 58 | |
| +86-0551-65418684 +8618949823763 |
China | 25356 | 58 |