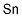
Тин
- английское имяTin
- CAS №7440-31-5
- CBNumberCB3190047
- ФормулаSn
- мольный вес118.71
- EINECS231-141-8
- номер MDLMFCD00133862
- файл Mol7440-31-5.mol
| Температура плавления | 231.9 °C (lit.) |
| Температура кипения | 2270 °C (lit.) |
| плотность | 7.310 g/mL at 25 °C (lit.) |
| давление пара | 1Pa at 1223.85℃ |
| Fp | 2270°C |
| температура хранения | no restrictions. |
| растворимость | H2O: soluble |
| форма | wire |
| цвет | Silvery-gray |
| Удельный вес | 7.31 |
| удельное сопротивление | 11 μΩ-cm, 20°C |
| Растворимость в воде | reacts slowly with cold dilute HCl, dilute HNO3, hot dilute H2SO4; readily with conc HCl, aqua regia [MER06] |
| Кристальная структура | Cubic, Alpha-Tin; Diamond Structure - Space Group Fd3m |
| Мерк | 13,9523 |
| Пределы воздействия | ACGIH: Ceiling 2 ppm OSHA: Ceiling 5 ppm(7 mg/m3) NIOSH: IDLH 50 ppm; Ceiling 5 ppm(7 mg/m3) |
| Стабильность | Stable. Incompatible with strong oxidizing agents. Highly flammable as a powder. Can, in powder form, lead to dust explosions. Moisture sensitive. |
| ИнЧИКей | OLGIDLDDXHSYFE-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 7440-31-5(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 5 |
| FDA UNII | 387GMG9FH5 |
| Система регистрации веществ EPA | Tin (7440-31-5) |
| Коды опасности | Xi,F,C | |||||||||
| Заявления о рисках | 36/37/38-36/37-11-36/38-34-20/21/22 | |||||||||
| Заявления о безопасности | 26-24/25-22-36/37/39-33-16-36/37-45 | |||||||||
| РИДАДР | UN 3264 8/PG 2 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 2 mg/m3 [*Note: The REL also applies to other inorganic tin compounds (as Sn) except tin oxides.] | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | XP7320000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 4.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 80070080 | |||||||||
| Банк данных об опасных веществах | 7440-31-5(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H228:Воспламеняющееся твердое вещество.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P241:Использовать взрывобезопасное оборудование и освещение.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Тин химические свойства, назначение, производство
Описание
Tin has a long, colorful history. The extraction and use of tin began during the Bronze Age around 3000 BC when early craftsmen discovered that bronze – a noncorrosive metal that is extremely hard and strong enough to be used for spears, swords, arrows, and other especially important objects at that time – could be produced by smelting tin with copper. Tin is also the primary constituent of pewter. Long ago, people developed the belief that trace amounts of tin seemed to help prevent fatigue and depression, and that drinking out of tin cups could help combat these ailments. Tin toys, tin coated cans, and tin roofs have also enjoyed great popularity in the past.Химические свойства
Tin is a gray to almost silver-white, ductile, malleable, lustrous metal.Физические свойства
Tin is a soft, silvery-white metal located in the carbon group, similar in appearance to freshcutaluminum. When polished, it takes on a bluish tint caused by a thin protective coatingof oxidized tin. This property makes it useful as a coating for other metals. It is malleable andductile, meaning it can be pounded, rolled, and formed into many shapes, as well as “pulled”into wires through a die.There are two allotropes of tin. One is known as gray or alpha (α) tin, which is not verystable. The other is known as white tin or beta (β), which is the most common allotrope. Thetwo forms (allotropes) of tin are dependent on temperature and crystalline structure. Whitetin is stable at about 13.2°C. Below this temperature, it turns into the unstable gray alphaform. There is also a lesser-known third allotrope of tin called “brittle tin,” which exists above161°C. Its name is derived from its main property.
Tin’s melting point is 231.93°C, its boiling point is 2,602°C, and the density is 5.75 g/cm3for the gray allotrope (alpha) and 7.287 g/cm3 for the white allotrope (beta).
Изотопы
There are 49 isotopes of tin, 10 of which are stable and range from Sn-112to Sn-124. Taken together, all 10 stable isotopes make up the natural abundance of tinfound on Earth. The remaining 39 isotopes are radioactive and are produced artificially innuclear reactors. Their half-lives range from 190 milliseconds to 1×10+5 years.Происхождение имени
The name “tin” is thought to be related to the pre-Roman Etruscan god Tinia, and the chemical symbol (Sn) comes from stannum, the Latin word for tin.Вхождение
Tin is the 49th most abundant element found in the Earth’s crust. Although tin is nota rare element, it accounts for about 0.001% of the Earth’s crust. It is found in deposits inMalaysia, Thailand, Indonesia, Bolivia, Congo, Nigeria, and China. Today, most tin is minedas the mineral ore cassiterite (SnO2), also known as tinstone, in Malaysia. Cassiterite is tin’smain ore. There are no significant deposits found in the United States, but small deposits arefound on the southeast coast of England. To extract tin from cassiterite, the ore is “roasted” ina furnace in the presence of carbon, thereby reducing the metal from the slag.Характеристики
Although tin is located in group 14 as a metalloid, it retains one of the main characteristicsof metals: in reacting with other elements, it gives up electrons, forming positive ions just asdo all metals.Tin has a relatively low melting point (about 231°C or 4,715°F), and it reacts with someacids and strong alkalis, but not with hot water. Its resistance to corrosion is the main characteristicthat makes it a useful metal.
There is an interesting historical event related to the two main allotropes of tin. At temperaturesbelow 13 degrees centigrade, “white” tin is slowly transformed into “gray” tin, whichis unstable at low temperatures, and during the brutally cold winter of 1850 in Russia, thetin buttons sewn on soldiers’ uniforms crumbled as the tin changed forms. In the 1800s, tinwas also widely used for pots, pans, drinking cups, and dinner flatware. However, at very lowtemperatures, these implements also disintegrated as their chemical structure was altered.
Использование
Chiefly for tin-plating and manufacture of food, beverage and aerosol containers, soldering alloys, babbitt and type metals, manufacture of tin salts, collapsible tubes, coating for copper wire. Principle component in pewter. Alloys as dental materials (silver-tin-mercury), nuclear reactor components (tin-zirconium), aircraft components (tin-titanium), bronze (copper-tin), brass.Методы производства
Tin is relatively rare, composing only about 0.0006% in the earth’s crust. The major tin ore is cassiterite, a naturally occurring tin (IV) oxide (SnO2). The other major tin-containing minerals are stannate, teallite, cylindrite, and canfieldite that are sulfides of tin.Определение
Metallic element of atomic number 50, group IVA of the periodic system, aw 118.69, valences of 2, 4; 10 isotopes.Общее описание
White TIN is an almost silver-white, ductile, malleable, lustrous solid. Mp 232°C; bp: 2507°C. Density: 7.3 g cm-3. Pure white TIN becomes non-metallic powdery gray TIN if held for a sustained period at temperatures less than 13°C.Профиль реактивности
TIN is a reducing agent. Stable in massive form in air, but oxidizes (corrodes) in air as a powder, especially in the presence of water. Dissolve slowly in dilute strong acids in the cold. Dissolves in hot aqueous KOH and other strongly basic solutions. Incompatible with acids and base. Incompatible with chlorine and turpenTINe.Опасность
Tin, as the elemental metal, is nontoxic. Most, but not all of tin’s inorganic salts and compoundsare also nontoxic.In contrast, almost all organic tin compounds (tin compounds composed of carbon andhydrocarbons) are very toxic and should be avoided. If they are used, special equipment andcare must be taken in handling.
(Note: When chemical formulas use the letter “R” preceding an element’s symbol, it designatessome form of organic compound—for example, R4Sn. If the letter “X” follows theelement’s symbol in a formula, it designates some form of inorganic compound—for example,SnX2. Thus, a whole series of tin compounds could be designated as R4Sn2, R2Sn, or SnX4,SnX2, and so forth.)
Угроза здоровью
Inorganic tin salts are irritants of the eyes and skin. No systemic effects have been reported from industrial exposure. Some inorganic tin compounds can cause skin or eye irritation because of acid or alkaline reaction produced with water. Tin tetrachloride, stannous chloride, and stannous sulfate are strong acids; sodium and potassium stannate are strong alkalies.Промышленное использование
Hot-dip coatings can be applied to fabricatedparts made of mild and alloy steels, cast iron,and copper and copper alloys to improveappearance and corrosion resistance. Like zinc,the coatings consist of two layers — a relativelypure outer layer and an intermediate alloy layer.An invisible surface film of stannic oxideis formed during exposure, which helps toretard, but does not completely prevent, corrosion.The coatings have good resistance to tarnishingand staining indoors, and in most rural,marine, and industrial atmospheres. They alsoresist foods. Corrosion resistance in all casescan be markedly improved by increasing thicknessand controlling porosity. Typical applicationswhere they can be used are milk cans,condenser and transformer cans, food and beveragecontainers, and various items of sanitaryequipment such as cast iron mincing machinesand grinders.
Профиль безопасности
An inhalation hazard. Questionable carcinogen with experimental tumorigenic data by implant route. Combustible in the form of dust when exposed to heat or by spontaneous chemical reaction with Br2, BrF3, Cl2, ClF3, Cu(NO3), K2O2, S. See also POWDERED METALS and TIN COMPOUNDS.Возможный контакт
The most important use of tin is as a protective coating for other metals, such as in the food and beverage canning industry; in roofing tiles; silverware, coated wire; household utensils; electronic components; and pistons. Common tin alloys are phosphor bronze; light brass; gun metal; high tensile brass; manganese bronze; die-casting alloys; bearing metals; type metal; and pewter. These are used as soft solders, fillers in automobile bodies; and as coatings for hydraulic brake parts; aircraft landing gear and engine parts. Metallic tin is used in the manufacture of collapsible tubes and foil for packaging. Exposures to tin may occur in mining, smelting, and refining; and in the production and use of tin alloys and solders. Inorganic tin compounds are important industrially in the production of ceramics; porcelain, enamel, glass; and inks; in the production of fungicides; anthelmintics, insecticides; as a stabilizer it is used in polyvinyl plastics and chlorinated rubber paints; and it is used in plating baths.Канцерогенность
Limited animal testing with stannous chloride has not revealed evidence of carcinogenic potential. Mixed results have been observed in genotoxic assays.Перевозки
UN3089 Metal powders, flammable, n.o.s., Hazard Class: 4.1; Labels: 4.1-Flammable solid.Методы очистки
Tin powder is purified by adding it to about twice its weight of 10% aqueousNaOH and shaking vigorously for 10minutes. (This removes oxide film and stearic acid or similar material that is sometimes added for pulverisation.) It is then filtered, washed with water until the washings are no longer alkaline to litmus, rinsed with MeOH and dried in air. [Sisido et al. J Am Chem Soc 83 538 1961.]Несовместимости
TIN is a reducing agent. Stable in bulk form in air, but as powder it corrodes (oxidizes) in air, especially in the presence of moisture. Keep away from strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Incompatible with acids, alkalies, bases, chlorine, turpentine; reacts violently with acetic aldehyde, ammonium nitrate, ammonium perchlorate, hexachloroethane. Strong reducing agents may react violently with halogens, bromine fluoride, chlorine trifluoride, copper nitrate, disulfur dichloride, nitrosyl fluoride, potassium dioxide, sodium peroxide, sulfur, and other chemicals. May form explosive compounds with hexachloroethane, pentachloroethane, picric acid, potassium iodate, potassium peroxide, 2,4,6-trinitrobenzene-1,3,5-triol.Тин запасные части и сырье
сырьё
запасной предмет
- 2 - (2-тиенил) пирролидин
- 5,7-дихлороксазоло[5,4-d]пиримидин
- Stannous Chloride Dihydrate extra
- 6-гидроксинафталин-2-бороновой кислоты
- Stannous octoate
- 3,4-диаминотиофен дигидрохлорид
- 7-hydroxyquinazolin-4(3H)-one
- Нафтол AS-BI фосфат
- Azocyclotin
- Дибутилолова оксид
- 2,4,5-Trimethoxyaniline
- Олово(IV) хлорид
- Пиреноксин
- 4-chloroquinazolin-7-ol
- 2-Chloro-1,4-diaminobenzene
- 4-ТИОФЕН-2-ИЛФЕНИЛАМИН
- 4-аминобензамидин дигидрохлорид
- Натрий тетрафторборат
- 4-(бромметил)-2,1,3-бензотиадиазол
- Fenbutatin oxide
- этил-2-(4-хлорхиназолин-7-илокси)ацетат
- ОКСИД ОЛОВА (II)
- Azocyclotin suspensoid
- Tin fluoroborate
- ДИАМИН 2,3,5-ТРИМЕТИЛ-1,4-БЕНЗОЛА
1of7
Тин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-17736087130 +86-18633844644 |
China | 985 | 58 | |
| +86-18186686046 +86-18186686046 |
China | 5820 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21636 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29885 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 18631714998 | CHINA | 904 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49374 | 58 | |
| 18503026267 | CHINA | 9636 | 58 |