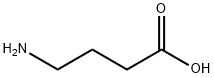
4-аминомасляная кислота
- английское имя4-Aminobutyric acid
- CAS №56-12-2
- CBNumberCB3184042
- ФормулаC4H9NO2
- мольный вес103.12
- EINECS200-258-6
- номер MDLMFCD00008226
- файл Mol56-12-2.mol
химическое свойство
| Температура плавления | 195 °C (dec.) (lit.) |
| Температура кипения | 248.0±23.0 °C(Predicted) |
| плотность | 1.2300 (estimate) |
| показатель преломления | 1.4650 (estimate) |
| FEMA | 4288 | 4-AMINOBUTYRIC ACID |
| температура хранения | 2-8°C |
| растворимость | H2O: 1 M at 20 °C, clear, colorless |
| форма | Powder |
| пка | 4.031(at 25℃) |
| цвет | White to almost white |
| Запах | at 1.00 % in propylene glycol. savory meaty |
| Odor Type | meaty |
| Растворимость в воде | SOLUBLE |
| Мерк | 14,430 |
| Номер JECFA | 1771 |
| БРН | 906818 |
| ИнЧИКей | BTCSSZJGUNDROE-UHFFFAOYSA-N |
| LogP | -3.17 |
| Справочник по базе данных CAS | 56-12-2(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | 4-AMINOBUTYRIC ACID |
| FDA UNII | 2ACZ6IPC6I |
| Справочник по химии NIST | 4-Aminobutanoic acid(56-12-2) |
| Система регистрации веществ EPA | 4-Aminobutanoic acid (56-12-2) |
| UNSPSC Code | 85151701 |
| NACRES | NA.32 |
больше
| Коды опасности | Xi,Xn | |||||||||
| Заявления о рисках | 36/37/38-20/21/22 | |||||||||
| Заявления о безопасности | 26-36 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | ES6300000 | |||||||||
| Примечание об опасности | Irritant | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29224995 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H302:Вредно при проглатывании.
H332:Вредно при вдыхании.
H312:Вредно при попадании на кожу.
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
4-аминомасляная кислота химические свойства, назначение, производство
Описание
4-Aminobutyric acid (GABA) is the chief inhibitory neurotransmitter in the mammalian central nervous system. It plays a role in regulating neuronal excitability throughout the nervous system. In humans, GABA is also directly responsible for the regulation of muscle tone. Although chemically it is an amino acid, GABA is rarely referred to as such in the scientific or medical communities, because the term "amino acid," used without a qualifier, conventionally refers to the alpha amino acids, which GABA is not, nor is it ever incorporated into a protein. In spastic diplegia in humans, GABA absorption becomes impaired by nerves damaged from the condition's upper motor neuron lesion, which leads to hypertonia of the muscles signaled by those nerves that can no longer absorb GABA.Химические свойства
4-Aminobutyric acid is a white flake or needle-like crystal; slightly odorous, deliquescence; easily soluble in water, slightly soluble in hot ethanol, insoluble in cold ethanol, ether and benzene; decomposition point is 202°C; LD50 (rat, abdominal cavity) 5400mg/kg.История
4-Aminobutyric acid was first synthesized in 1883, and was first known only as a plant and microbe metabolic product. In 1950, however, GABA was discovered to be an integral part of the mammalian central nervous system.Использование
4-Aminobutyric acid is an important inhibitory neurotransmitter in the central nervous system, which has good water solubility and thermal stability. It has been confirmed that GABA, as a small molecular weight non protein amino acid, has edible safety and can be used in the production of beverages and other foods. Studies have shown that a certain amount of GABA can improve the body's sleep quality and reduce blood pressure.The foods contain γ-aminobutyric acid (GABA) at an amount that shows immediate effect of suppressing autonomic nerve activity related to blood pressure increase. Reacts with isothiocyanates to produce thioureas which have antifungal activity.Подготовка
The synthesis of 4-aminobutyric acid mainly includes the following: the first is the use of potassium Phthaloyl imine and γ- Chloroprene cyanogen or butyrolactone is used as the raw material of GABA. The final product obtained after violent reaction and hydrolysis is GABA; The second is to use pyrrolidone as the initial raw material, hydrolyze it through calcium hydroxide and ammonium bicarbonate, and finally open its ring to obtain GABA; The third is to use butyric acid and ammonia as raw materials of GABA γ GABA was obtained by light reaction under X-ray conditions; The fourth method is to synthesize GABA with propylamine and formic acid by glow discharge; The fifth is to use methyl bromoacetate and ethylene as raw materials to prepare GABA. Methyl 4-bromobutyrate is obtained through polymerization. Finally, the product after ammonolysis and hydrolysis is GABA. The chemical synthesis methods of GABA have the disadvantages of difficult reaction control and high cost.Определение
4-Aminobutyric acid is a gamma-amino acid that is butanoic acid with the amino substituent located at C-4. It has a role as a signalling molecule, a human metabolite, a Saccharomyces cerevisiae metabolite and a neurotransmitter. It is a gamma-amino acid and a monocarboxylic acid. It derives from a butyric acid. It is a conjugate acid of a gamma-aminobutyrate. It is a tautomer of a gamma-aminobutyric acid zwitterion.Общее описание
4-Aminobutyric acid is a chief inhibitory neurotransmitter, which is found in the cerebellum, hypothalamus, thalamus and hippocampus. It is formed via the decarboxylation of L-glutamate catalyzed by the enzyme, glutamic acid decarboxylase(GAD).Механизм действия
4-Aminobutyric acid (GABA) probably represents the most important inhibitory transmitter of the mammalian CNS. Both types of GABAergic inhibition (pre- and postsynaptic) use the same GABAA receptor subtype, which acts by regulation of the chloride channel of the neuronal membrane. A second GABA receptor type, GABAB, that is a G protein–coupled receptor is not considered to be important in understanding the mechanism of hypnotics. Activation of a GABAA receptor by an agonist increases the inhibitory synaptic response of central neurons to GABA through hyperpolarization. Because many, if not all, central neurons receive some GABAergic input, this leads to a mechanism by which CNS activity can be depressed. For example, if the GABAergic interneurons are activated by an agonist that inhibits the monoaminergic structures of the brainstem, hypnotic activity will be observed. The specific neuronal structures in different brain regions affected by GABAA agonist continues to be better defined.Фармаколо?гия
Drugs that act as allosteric modulators of GABA receptors (known as GABA analogues or GABAergic drugs) or increase the available amount of GABA typically have relaxing, anti-anxiety, and anti-convulsive effects. Many of the substances below are known to cause anterograde amnesia and retrograde amnesia.In general, GABA does not cross the blood–brain barrier, although certain areas of the brain that have no effective blood–brain barrier, such as the periventricular nucleus, can be reached by drugs such as systematically injected GABA. At least one study suggests that orally administered GABA increases the amount of Human Growth Hormone. GABA directly injected to the brain has been reported to have both stimulatory and inhibitory effects on the production of growth hormone, depending on the physiology of the individual.
Источник
4-Aminobutyric acid can be found in various foods, including:Tea
Tomatoes
Soybeans
Germinated rice
Some fermented foods, such as tempe and kimchi
Some vegetables, such as broccoli, spinach, and kale
GABA is also widely available as a supplement in either powder or pill form.
Метаболизм
GABA transaminase enzyme catalyzes the conversion of 4- aminobutanoic acid and 2-oxoglutarate into succinic semialdehyde and glutamate. Succinic semialdehyde is then oxidized into succinic acid by succinic semialdehyde dehydrogenase and as such enters the citric acid cycle as a usable source of energy.Методы очистки
Crystallise GABA from aqueous EtOH or MeOH/Et2O. Also crystallise it by dissolving it in the least volume of H2O and adding 5-7 volumes of absolute EtOH.4-аминомасляная кислота запасные части и сырье
4-аминомасляная кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 571-88938639 +8617705817739 |
China | 52849 | 58 | ||
| 029-86107037 13289246953 |
China | 1169 | 58 | ||
| 0592-5800732; +8613806035118 |
China | 988 | 58 | ||
| +8615669938129 | China | 130 | 58 | ||
| +8618629063126 | China | 435 | 58 | ||
| +86-027-59207850 | China | 5972 | 58 | ||
| +86-18532138899 +86-18532138899 |
China | 939 | 58 | ||
| +86-19925801629 +86-19925801629 |
China | 242 | 58 | ||
| +86-86-13343427080 +8613343427080 |
China | 456 | 58 | ||
| 0531-88110457; +8615562555968 |
China | 260 | 58 |