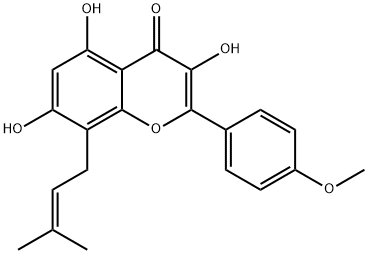
Icaritin
- русский язык имя
- английское имяIcaritin
- CAS №118525-40-9
- CBNumberCB2982508
- ФормулаC21H20O6
- мольный вес368.38
- номер MDLMFCD22422519
- файл Mol118525-40-9.mol
химическое свойство
| Температура плавления | 239℃ |
| Температура кипения | 582.0±50.0 °C(Predicted) |
| плотность | 1.359 |
| Fp | 206.7℃ |
| температура хранения | 2-8°C |
| растворимость | DMSO: soluble5mg/mL, clear (warmed) |
| форма | powder |
| пка | 6.44±0.40(Predicted) |
| цвет | light yellow to dark yellow |
| λмакс | 368nm(MeOH)(lit.) |
| InChI | InChI=1S/C21H20O6/c1-11(2)4-9-14-15(22)10-16(23)17-18(24)19(25)20(27-21(14)17)12-5-7-13(26-3)8-6-12/h4-8,10,22-23,25H,9H2,1-3H3 |
| ИнЧИКей | TUUXBSASAQJECY-UHFFFAOYSA-N |
| SMILES | C1(C2=CC=C(OC)C=C2)OC2=C(C/C=C(/C)\C)C(O)=CC(O)=C2C(=O)C=1O |
| FDA UNII | UFE666UELY |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
Icaritin химические свойства, назначение, производство
Химические свойства
Yellow crystalline powder, soluble in methanol, ethanol, DMSO and other organic solvents, derived from epimedium.Использование
Icaritin is a hydrolytic product of Icariin, a traditional Chinese herbal medicine extracted from the Epimedium genus. Icaritin and Desmethylicaritin, is two metabolites of Icariin, dramatically inhibit the growth of most malignant cells. They also have significant antiangiogenesis properties, inhibiting or eliminating entirely the development of new malignant cells. Icaritin has been used in trials studying the treatment of Solid Tumors, Metastatic Breast Cancer, and Hepatocellular Carcinoma (HCC).Синтез
The novel total synthesis of icaritin, naturally occurring with important bioactive 8-prenylflavonoid, was performed via a reaction sequence of 8 steps including Baker-Venkataraman reaction, chemoselective benzyl or methoxymethyl protection, dimethyldioxirane (DMDO) oxidation, O-prenylation, Claisen rearrangement and deprotection, starting from 2,4,6-trihydroxyacetophenone and 4-hydroxybenzoic acid in overall yields of 23%. The key step was Claisen rearrangement under microwave irradiation.Total Synthesis of Icaritin via Microwave-assistance Claisen Rearrangement
Способ действия
Icaritin is a flavonoid first isolated from the Chinese herb H. epimedii that demonstrates anticancer activity against a variety of tumor cell lines. Icaritin was shown in vitro to sustain extracellular signal–regulated kinase (ERK) activity through stimulating estrogen receptors. Prolonged ERK activation arrested the cell cycle in the G2 stage and subsequently triggered apoptosis. It has been shown to inhibit fatty acid synthase, reducing IGF-1-induced activation of STAT3 in several melanoma cell lines.Icaritin запасные части и сырье
сырьё
1of2
запасной предмет
Icaritin поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8618829768577 | China | 1249 | 58 | ||
| +8618516098983 | China | 1001 | 58 | ||
| +86-18791163155 +86-13119157289 |
China | 3558 | 58 | ||
| +undefined18602966907 | China | 997 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 008657128800458; +8615858145714 |
China | 7738 | 55 | ||
| 18017610038 | CHINA | 3619 | 58 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| 028-87075086 13350802083 |
CHINA | 1824 | 58 |