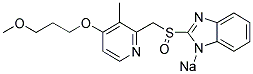
Rabeprazole Sodium
- русский язык имя
- английское имяRabeprazole Sodium
- CAS №117976-90-6
- CBNumberCB2420819
- ФормулаC18H21N3O3S.Na
- мольный вес382.43
- EINECS629-730-3
- номер MDLMFCD02092688
- файл Mol117976-90-6.mol
химическое свойство
| Температура плавления | 140-141°C dec. |
| температура хранения | -20°C |
| растворимость | H2O: soluble10mg/mL (clear solution) |
| форма | powder |
| цвет | white to beige |
| Растворимость в воде | H2O: 10mg/mL (clear solution) |
| Мерк | 14,8089 |
| Стабильность | Hygroscopic |
| InChI | InChI=1S/C18H20N3O3S.Na/c1-13-16(19-9-8-17(13)24-11-5-10-23-2)12-25(22)18-20-14-6-3-4-7-15(14)21-18;/h3-4,6-9H,5,10-12H2,1-2H3;/q-1;+1 |
| ИнЧИКей | KRCQSTCYZUOBHN-UHFFFAOYSA-N |
| SMILES | O=S(CC1C(C)=C(OCCCOC)C=CN=1)C1[N-]C2=CC=CC=C2N=1.[Na+] |
| FDA UNII | 3L36P16U4R |
| Словарь наркотиков NCI | rabeprazole sodium |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
H413:Может вызвать долгосрочные отрицательные последствия для водных организмов.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P273:Избегать попадания в окружающую среду.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Rabeprazole Sodium химические свойства, назначение, производство
Описание
Rebeprazole sodium was launched as Pariet in Japan, its first market, for the treatment of peptic ulcers including gastric and duodenal ulcers. From 4-chloro- 2,3-dimethylpyridine N-oxide, a six step synthesis allows access to the basic skeleton after successive condensations. Rabeprazole, a structural analog of Omeprazole, the first compound to have been marketed in this class up to now, is reported to be a more potent inhibitor of gastric H+/K+-adenosine triphosphate (ATPase) ; a common mechanism of action of this chemical class involves the conversion at low pH to a reactive sulphonamide that itself binds to cysteine residues located on the enzyme. Moreover, rabeprazole showed an antibacterial activity against Helicobacter Pylori, with a MIC90 of 1.56 μg/ml.Rebeprazole sodium has a faster onset of action compared with omeprazole, but a shorter duration of action, being extensively and rapidly metabolized in several animal species. In clinical studies in patients with gastric ulcers, 10 and 20 mg rabeprazole sodium once-daily significantly inhibited basal and stimulated acid output. Rabeprazole is awaiting registration in the US for treatment of gastrooesophageal reflux disease (GORD) and other pathologic hypersecretory conditions including Zollinger-Ellison syndrome.
Химические свойства
Rebeprazole sodium is White Crystalline SolidИспользование
Rebeprazole sodium is a partially reversible gastric proton pump inhibitor. It belongs to a national second-class drug that can be used for the treatment of gastric ulcers. It can treat peptic ulcer, gastroesophageal reflux disease, zollinger-ellison syndrome and other diseases. This compound has the effects of anthelminthic, antiseptic, and expectorant. As an antibacterial and antifungal agent, it is effective against gram-positive and gram-negative bacteria, yeast and fungi.Общее описание
Rabeprazole sodium, 2[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]methyl]sulfinyl]-1H-benzimidazole sodium salt (Aciphex), is a white toslightly yellowish white solid. It is very soluble in water andmethanol, freely soluble in ethanol, chloroform, and ethyl acetate,and insoluble in ether and hexane. Rabeprazole is aweak base (pyridine N, pKa 4.53) and a weak acid (benzimidazoleN-H, pKa 0.62), faciliting sodium salt formation.Rabeprazole sodium is formulated as enteric-coated,delayed-release tablets to allow the drug to pass throughthe stomach relatively intact. After oral administration of20-mg peak plasma concentrations (Cmax) occur over arange of 2 to 5 hours (Tmax). Absolute bioavailability for a20-mg oral tablet of rabeprazole (vs. IV administration) isapproximately 52%. The plasma half-life ofrabeprazole ranges from 1 to 2 hours. The effects of foodon the absorption of rabeprazole have not been evaluated.Rabeprazole is 96% bound to human plasma proteins.Rabeprazole is extensively metabolized in the liver. Thethioether and sulfone are the primary metabolites measuredin human plasma resulting from CYP3A oxidation.Additionally, desmethyl rabeprazole is formed via the actionof CYP2C19. Approximately 90% of the drug is eliminatedin the urine, primarily as thioether carboxylic acidand its glucuronide and mercapturic acid metabolites. Theremainder of the dose is recovered in the feces. No unchangedrabeprazole is excreted in the urine or feces.
Rabeprazole Sodium запасные части и сырье
Rabeprazole Sodium поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8613132578992 | China | 251 | 58 | ||
| +86-19925801629 +86-19925801629 |
China | 242 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| 18758118018 | China | 255 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +8613288715578 | China | 1174 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-21-33585366 - 03@ | CHINA | 738 | 60 |