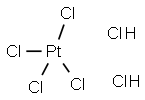
Chloroplantinic acid
- русский язык имя
- английское имяChloroplantinic acid
- CAS №16941-12-1
- CBNumberCB2271499
- ФормулаCl6H2Pt
- мольный вес409.81
- EINECS241-010-7
- номер MDLMFCD00011330
- файл Mol16941-12-1.mol
| Температура плавления | 60 °C(lit.) |
| плотность | 2.43 g/mL at 25 °C(lit.) |
| давление пара | 0Pa at 20℃ |
| показатель преломления | n |
| температура хранения | 2-8°C |
| растворимость | H2O: 0.5 M at 20 °C, clear, orange |
| форма | powder and chunks |
| цвет | orange |
| Растворимость в воде | soluble |
| Мерк | 14,7526 |
| Пределы воздействия | ACGIH: TWA 0.002 mg/m3 NIOSH: IDLH 4 mg/m3; TWA 0.002 mg/m3 |
| Стабильность | May decompose on exposure to light, air or moisture. |
| ИнЧИКей | GBFHNZZOZWQQPA-UHFFFAOYSA-J |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | Q65224GJ7F |
| Система регистрации веществ EPA | Platinate(2-), hexachloro-, hydrogen (1:2), (OC-6-11)- (16941-12-1) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | T,C,Xi | |||||||||
| Заявления о рисках | 25-34-42/43-36/38-36/37/38-22 | |||||||||
| Заявления о безопасности | 26-27-36/37/39-45-22-23 | |||||||||
| РИДАДР | UN 3264 8/PG 3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | TP1510000 | |||||||||
| F | 3-8-10 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28439000 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H411:Токсично для водных организмов с долгосрочными последствиями.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H334:При вдыхании может вызывать аллергическую реакцию (астму или затрудненное дыхание).
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
H290:Может вызывать коррозию металлов.
-
оператор предупредительных мер
P234:Хранить только в оригинальной упаковке.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Chloroplantinic acid химические свойства, назначение, производство
Химические свойства
Orange/Red CrystalsИспользование
Chloroplantinic acid (H2PtCl6) is one of the most commercially important compounds of platinum. Its many uses include etching on zinc, making indelible ink, plating, and coloring in fine porcelains and use in photography, in mirrors, and as a catalyst.Определение
chloroplatinic acid: A reddish crystallinecompound, H2PtCl6, made bydissolving platinum in aqua regia.Общее описание
Chloroplatinic acid, is a reddish-brown solid. Chloroplatinic acid is soluble in water and will yield a mildly acidic solution. Chloroplatinic acid may cause illness from inhalation of the dust and Chloroplatinic acid is irritating to skin and eyes. When heated to high temperatures Chloroplatinic acid may decompose to toxic chloride fumes. Chloroplatinic acid may burn, but may be difficult to ignite. Chloroplatinic acid is used for manufacturing indelible ink and in electroplating processes.Реакции воздуха и воды
Soluble in water.Профиль реактивности
Oxidizing acids are generally soluble in water with the release of hydrogen ions. The resulting solutions have pH's of less than 7.0. Materials in this group react with chemical bases (for example: amines and inorganic hydroxides) to form salts. These neutralization reactions occur as the base accepts hydrogen ions that the acid donates. Neutralizations can generate dangerously large amounts of heat in small spaces. The dissolution of acids in water or the dilution of their concentrated solutions with water may generate significant heat. The addition of water acids often generates sufficient heat in the small region of mixing to boil some of the water explosively. The resulting "bumping" spatters acid widely. These materials have significant ability as oxidizing agents. but that ability varies (for example, from high for nitric acid to low for sulfuric acid and most sulfonic acids). They can react with active metals, including iron and aluminum, and also many less active metals, to dissolve the metal and liberate hydrogen and/or toxic gases. Like other acids, materials in this group can initiate polymerization in certain classes of organic compounds. Their reactions with cyanide salts and compounds release gaseous hydrogen cyanide. Flammable and/or toxic gases are also often generated by their reactions with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and weak or strong reducing agents. Additional gas-generating reactions occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and even carbonates: the carbon dioxide gas from the last is nontoxic but the heat and spattering from the reaction can be troublesome. Acids often catalyze (increase the rate) of chemical reactions.Угроза здоровью
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Профиль безопасности
Poison by intravenous and intraperitoneal routes. Mutation data reported. See PLATINUM COMPOUNDS and CHLORIDES. Incompatible with BrF3. When heated to decomposition it emits toxic fumes of Cl-.Возможный контакт
Chloroplatinic acid has many uses, among them are platinum plating, photography, and catalysis.Перевозки
UN2507 Chloroplatinic acid, solid, Hazard class: 8; Labels: 8-Corrosive material.Методы очистки
If it is to be purified, or regenerated from Pt recovered from catalytic hydrogenations, it should be dissolved in aqua regia followed by evaporation to dryness and dissolution in the minimum volume of H2O. Then the aqueous solution is treated with saturated ammonium chloride until all the ammonium hexachloroplatinate separates. The (NH4)2PtCl6 is filtered off and dried at 100o. Igniting this salt gives Pt sponge; dissolve the Pt sponge in aqua regia, boil to dryness, dissolve the residue in concentrated HCl, boil to dryness again and repeat the process. Protect it from light. [Hickers J Am Chem Soc 43 1268 1921, Adams et al. Org Synth Coll Vol I 463, 466 1941, Bruce J Am Chem Soc 58 687 1936.]Несовместимости
Oxidizing acids are generally soluble in water with the release of hydrogen ions. The resulting solutions have pH’s of <7.0. Materials in this group react with chemical bases (e.g., amines and inorganic hydroxides) to form salts. These neutralization reactions occur as the base accepts hydrogen ions that the acid donates. Neutralizations can generate dangerously large amounts of heat in small spaces. The dissolution of acids in water or the dilution of their concentrated solutions with water may generate significant heat. The addition of water acids often generates sufficient heat in the small region of mixing to boil some of the water explosively. The resulting “bumping” spatters acid widely. These materials have significant ability as oxidizing agents. but that ability varies (e.g., from high for nitric acid to low for sulfuric acid and most sulfonic acids). They can react with active metals, including iron and aluminum, and also many less active metals, to dissolve the metal and liberate hydrogen and/or toxic gases. Like other acids, materials in this group can initiate polymerization in certain classes of organic compounds. Their reactions with cyanide salts and compounds release gaseous hydrogen cyanide. Flammable and/or toxic gases are also often generated by their reactions with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and weak or strong reducing agents. Additional gas-generating reactions occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and even carbonates: the carbon dioxide gas from the last is nontoxic but the heat and spattering from the reaction can be troublesome. Acids often catalyze (increase the rate) of chemical reactions.Chloroplantinic acid запасные части и сырье
сырьё
запасной предмет
Chloroplantinic acid поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-86-02137122233 +8613795318958 |
China | 299 | 55 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 | ||
| +undefined-21-51877795 | China | 32965 | 60 | ||
| 323-480-4688 | United States | 989 | 55 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| 86+18663751872 | CHINA | 491 | 58 | ||
| +86-531-88989536 +86-15508631887 |
China | 2958 | 58 |